|
 |
- Search
| J. Electrochem. Sci. Technol > Volume 10(2); 2019 > Article |
|
Abstract
Ni-rich layered oxides Li(NixCoyMnz)O2 (x + y + z = 1) have been extensively studied in recent times owing to their high capacity and low cost and can possibly replace LiCoO2 in the near future. However, these layered oxides suffer from problems related to the capacity fading, thermal stability, and safety at high voltages. In this study, we use surface coating as a strategy to improve the thermal stability at higher voltages. The uniform and conformal Al2O3 coating on prefabricated electrodes using atomic layer deposition significantly prevented surface degradation over prolonged cycling. Initial capacity of 190, 199, 188 and 166 mAh g−1 is obtained for pristine, 2, 5 and 10 cycles of ALD coated samples at 0.2C and maintains 145, 158, 151 and 130 mAh g−1 for high current rate of 2C in room temperature. The two-cycle Al2O3 modified cathode retained 75% of its capacity after 500 cycles at 5C with 0.05% capacity decay per cycle, compared with 46.5% retention for a pristine electrode, at an elevated temperature. Despite the insulating nature of the Al2O3 coating, a thin layer is sufficient to improve the capacity retention at a high temperature. The Al2O3 coating can prevent the detrimental surface reactions at a high temperature. Thus, the morphology of the active material is well-maintained even after extensive cycling, whereas the bare electrode undergoes severe degradation.
Since the first LiCoO2/graphite cell was fabricated by Sony in 1991, Li-ion batteries (LIBs) have dominated the portable energy market. To repeat its success story for large-scale applications, high-energy cathodes are necessary [1–3]. LIBs based on Li[NiXCoYAl1-X-Y]O2 could satisfy the theoretical energy density and are used commercially in the Tesla S. Next-generation electric vehicles (EVs) are highly dependent on Ni-rich layered oxides owing to the high discharge capacity of over 220 mAh g−1 and the low cost, which could certainly increase the energy density to satisfy the future driving range of EVs [4,5a,5b]. However, with the increasing Ni concentration, the material is prone to several drawbacks. The trace amount of moisture in the electrolyte vigorously reacts with LiPF6 and produces acidic HF, leading to dissolution of Li(NixCoyMnz)O2, especially at high temperatures [6]. Structural stability is a serious concern for these systems at high temperatures, owing to the parasitic surface reactions and the formation of an NiO-like phase from the reduction of highly reactive Ni4+ in the highly delithiated state and oxygen release that destabilizes the structure [7]. Micro-cracks generated from the surface of the primary particles affect the overall electrochemical performance of these layered oxides. This results in the inner core of these layered oxides being exposed to unwanted side reactions, with the electrolyte thereby significantly changing the morphology after extensive cycling [8]. Micro-cracks can also generate severe degradation mechanisms, as well as other mechanisms such as phase transition. Extensive research shows that structural degradation is initiated at the surface, thereby increasing the charge-transfer resistance [9]. Hence, a stable surface/interface is necessary for the cathodes in prolonged cycle life.
To improve the electrode/electrolyte interface, different approaches have been proposed, such as surface modification [10], foreign ion substitution [11,12], the core-shell structure [13], and electrolyte additives [14]. Among these, surface modification is highly successful in mitigating the irreversible side reactions and preventing structural collapse for long cycles [10]. Thus far, metal oxides [15–21] (Al2O3, ZrO2, MgO, CeO2, ZnO, La2O3, TiO2, SiO2, etc.), phosphates [22,23a,23b] (AlPO4, FePO4, Ni3(PO4)2) and fluorides [24,25] (AlF3, CaF2, etc.) are mostly investigated for layered oxides. Most of the aforesaid coatings were applied in a solution process. Specifically, amphoteric metal oxides such as Al2O3 are active HF scavengers, as follows: Al2O3+6HF →2AlF3+3H2O [15,16]. For example Al2O3-coated LiNi0.5Co0.2Mn0.3O2 exhibited high capacity retention of 85% after 100 cycles compared with the pristine electrode (only 75%). Atomic layer deposition (ALD) is a non-solution-based coating technique for conformal coating of thin films and surface layers down to atomic thickness with homogeneity, a wellcontrollable thickness, and high device performance [26]. ALD has become an attractive technique for energy-storage applications owing to its multi-functional capabilities for developing advanced electrode materials. Another salient feature of this technique is that it can be performed at very low temperatures (less than 300°C and even room temperature) on complex structures and three-dimensional substrates [27]. This widens its spectrum of application for temperature-sensitive substrates and biomedical applications [28]. Jung et al. studied the effect of an Al2O3 coating applied via ALD on the cathode and anode of LIBs [29,30]. Many other studies were performed on ALD coating on the cathode, anode, and separator, and the electrochemical performance was examined [30]. Previous works on ALD coating on LiCoO2 have shown positive outputs. Kim et al. applied an Al2O3 coating to NCM111 via ALD and thereby suppressed the charge-transfer impedance growth and capacity fading significantly for 3.0–4.5 V [31]. Yuno et al. created an outer covering of LiAlO2 using the sol-gel method and decreased the capacity fading between 2.5–4.7 V [32]. Mohanty et al. coated NCM811 and NCA particles directly with Al2O3 and TiO2 using ALD and studied them as pouch cells at different cutoff voltages [8]. However, only direct Al2O3 coating on active materials before electrode fabrication has been done thus far. This hinders the Li+ and electron conduction, resulting in poor kinetics at a high input current. Further, the thickness of the Al2O3 coating can have a significant effect on the electrochemical performance, and reports suggest that an optimized coating thickness is mandatory.
We studied the effect of different numbers of cycles of ALD Al2O3 coating on prefabricated NCM811 electrodes in the voltage range of 2.8–4.3 V at room temperature and a high temperature (50°C). Apparently, the Al2O3 coating improved both the initial capacity and the high-rate capability in both the room-temperature and elevated-temperature conditions. At the high temperature, the optimized ALD cycle (2) greatly improved the capacity retention to 75% under cycling at a rate of 5C up to 500 cycles. These results are meaningful for the development of NCM811 as a future cathode for EVs.
The electrochemical performance of all samples was studied using CR2032 coin cells, which were assembled inside a glovebox filled with ultrapure Ar, in which the O2 and H2O levels were maintained below 1 ppm. Commercial NCM811 (Ecopro, South korea) was used as a cathode, and Li metal was used as the anode. Whatman glass-fiber filter paper was used as a separator, and 1 M LiPF6 in EC:DMC (1:1) was used as the electrolyte solution. Each cathode consisted of 2.5 mg of the active material mixed with 0.5 mg of Ketjen black and 0.5 mg of Teflonized acetylene black (TAB-2). The slurry obtained with the assistance of ethanol was pressed on a stainless-steel current collector (200 mm2 area) and dried in a vacuum oven at 160°C for 4 h before cell fabrication. Charge/discharge studies were performed in the voltage range of 2.8–4.3 V with varying current rates of 85, 170, 340, 650, and 1,700 mA g−1 using an Arbin BT-2000 battery testing system. Cyclic voltammetry and impedance measurements were conducted using an electrochemical analyzer (SP-150, Biologic, France.)
A thin layer of Al2O3 film was deposited in a laminar-flow-type thermal ALD reactor (NCD, Lucida D100, Korea) on the prefabricated electrodes at the substrate temperature of 150°C using trimethylaluminum (TMA) and de-ionized-H2O as the Al and O sources, respectively. The TMA and H2O sources were kept at 10°C throughout the deposition process. A continuous flow of N2 gas (50 sccm) was used as the carrier gas and also for the purging during the deposition process. The reaction scheme for one Al2O3 ALD cycle was set as follows: 0.5 s pulsing of TMA, 20 s purging of N2, 0.5 s pulsing of H2O, and 20 s purging of N2. The repetition of the aforementioned sequence for two, five, and 10 cycles resulted in different thicknesses of the Al2O3 coating on the cathode materials. Hereinafter, the electrodes are denoted as NCM811-0c, NCM811-2c, NCM811-5c, and NCM811-10c, respectively.
The crystal structures were characterized via X-ray diffraction (XRD; Cu Ka radiation, Rint 1000, Rigaku, Japan) in the 2θ range of 5°–90°. The lattice parameters were determined by Rietveld refinement using the GSAS software. The particle morphology, elemental composition, and internal structure were evaluated using field-emission scanning electron microscopy (FE-SEM, S-4700, Hitachi, Japan), energy-dispersive X-ray spectroscopy, and high-resolution transmission electron microscopy (HR-TEM; JEM-2000, EX-II, JEOL, Japan), respectively. The stoichiometry was determined via inductively coupled plasma atomic emission spectroscopy analysis (Perkin Elmer, OPTIMA 8300, USA). XPS measurements (Multilab 2000, UK) were performed to determine the chemical oxidation sates of Mn, Ni, and Co on the compound surface.
Fig. 1 shows the structural and morphological properties of the NCM811 obtained from Ecopro (Korea) using XRD, FE-SEM, and TEM. As shown in Fig. 1(a), the well-defined XRD peaks represent the hexagonal α-NaFeO2 structure with the R-3m space group [33]. The peaks are further indexed using the hexagonal α-NaFeO2 structure. The absence of additional peaks and the splitting of (006)/(102) and (108)/(110) denote the phase purity of the sample, along with the well-defined layered structure. Fig. 1(b) shows the morphology of the single particle of as-obtained NCM811 with a distinct spherical shape as an advantage for a high tap density and an aid to formulate the quality electrodes. The uniform and conformal coating of ALD Al2O3 is confirmed by the magnified TEM image, as shown in Figs. 1(c and d). For the purpose of characterization, the electrodes are fabricated with less conductive C and coated with different thicknesses of ALD Al2O3. The particles at the surface of the cathode are removed properly and used for further analysis. For a 50-cycle ALD-coated electrode, an amorphous coating layer approximately 5 nm thick is clearly visible. This uniform coating protects the cathodes from electrolyte attack and improves the stability. Elemental distribution mapping was performed to confirm the distribution of individual elements over the surface. Fig. 2 confirms that Ni, Mn, Co and O are uniformly distributed over the particle. Further, the TEM elemental mapping of the 50-cycle ALD coating is presented in Fig. s1. A clear Al mapping is visible near the edge, confirming the uniformity of the Al2O3 coating corresponding to that observed in the TEM image.
Fig. 3(a) shows the charge-discharge curves of NCM811 electrodes modified with different thicknesses of the Al2O3 coating at a rate of 0.2C (1C = 200 mA g−1) within the voltage window of 2.8–4.3 V. It provides an initial capacity of ~190, ~199, ~188, and ~166 mAh g−1 for pristine, 2, 5, and 10 cycles of ALD coating. It is clear that the two-cycle ALD-coated electrode has a higher capacity than the pristine electrode. Further, the capacity is found to decrease linearly with the increasing coating thickness. Hence, an optimized coating thickness is necessary to realize better electrochemical performance. In the first place, an inactive protective matrix at the interface effectively prevents the cathode from direct contact with the electrolyte species and suppresses the side reactions [16]. Once the thickness of coating layer exceeds the threshold value, the Li+ ion and electron transport kinetics are severely affected [34a]. This property is material-sensitive and varies with different materials. The intensity ratio of (003)/(104) is an important factor to determine the degree of disorder in the layered oxides. It has been reported that, Al2O3 and AlPO4 surface coating resulted in increased (003)/(104) ratio for nickel rich layered oxides by preventing the cation mixing and having a much ordered surface structure. This results in lower activation barrier for diffusion of lithium in the layered structure. Thus optimized Al2O3 coating decreases the activation energy at the interface, thereby increasing the Li+ ion migration, leading to a high capacity [35]. Initial few cycles of ALD coating leads to partial coverage of Al at the interstitial sites of cathode electrode surface. The Aluminium ions from the Trimethyl Aluminium (TMA) precursor is tend to bond with the surface sites of the active materials and results in the graded incorporation of the Al into the structure through highly reactive and self-limiting chemistry. This justifies the improved electro-chemical performance of 2 cycle ALD modified NCM811 electrode at room temperature and elevated temperature. It also provides more channels for Li+ ion migration [36,46]. The corresponding differential capacity plot is given in Fig. 3(b). Three pairs of redox peaks are present in the voltage window of 2.8–4.3V for the bare electrode without the ALD coating. The sharp peaks centered at 3.75 V correspond to Ni2+-Ni4+, and another broad peak at 4.2 V correspond to Co3+-Co4+, along with other structural transitions similar to those of NCM811 [15]. For two-cycle ALD-modified electrodes, the Ni2+/4+ peaks are shifted to a lower voltage, and the separation between the peaks is minimized. This implies that the polarization and impedance are reduced. When the coating thickness is increased, the polarization is increased, resulting from the Li+/electron migration clearly reflected in the interfacial charge-transfer resistance derived from the impedance plot.
Rate capability studies were performed at different current densities between 2.8 and 4.3 V for pristine and ALD-modified electrodes (Fig. 3(c)). The NCM811 electrode without any surface modifications delivered 190, 208, 188, 183, 167, and 146 mAh g−1, at rates of 0.2, 0.1, 0.3, 0.5, 1, and 2C, respectively. When the cells were cycled back to a lower current of 0.2C, the electrode still delivered a capacity of 195 mAh g−1, showing good recovery characteristics. The discharge capacity decreases with the increasing current density owing to the lower diffusion rate of the cathode electrodes at a higher current rate. The rate performance of NCM811 with two-cycle Al2O3 coating was enhanced with 199, 214, 195, 189, 172, and 158 mAh g−1 for the aforementioned current rates, and the electrode exhibited a recovery capacity of 203 mAh g−1 when cycled at the rate of 0.2C. NCM811 with five cycles of ALD coating also gives a higher capacity than the pristine electrode, and as the thickness increases to 10 cycles, the rate performance degradation increases. This is because a thin ALD coating accelerates the formation of the stable interfacial layer with a more desirable structure, resulting in improved electrochemical kinetics [37]. In this case, the Al2O3 coating is directly applied on the prefabricated electrode without impeding the electrical conducting pathways between the active material and conductive C. As the thickness of the coating increases, the movement of Li+ is impeded, resulting in a poor rate capability [34]. Cyclic stability is an important factor for increasing the driving range of EVs. Here, the cells were tested at the rate of 1C between 2.8–4.3 V for 100 cycles (Fig. 3(d)). The pristine electrode delivered a capacity of 163 mAh g−1 at a rate of 1C rate and retained 151 mAh g−1 after 100 cycles with a capacity retention of 92.56%. In comparison, the two-cycle Al2O3-coated cathodes delivered 176 mAh g−1 and retained a capacity of 165 mAh g−1 (93.08%) after 100 cycles. The stability of cathodes significantly increases with a higher coating thickness, at the expense of the capacity. The fiveand ten-cycle ALD-coated electrode delivered a low capacity of 171 and 149 mAh g−1 and retained 96.75 and 98.45%, respectively, after 100 cycles. This results clearly show the improvement in the electrochemical performance of the ALD-modified Ni-rich cathodes. Li et al. applied an ALD coating to LiCoO2 electrodes using different metal oxides and confirmed the positive role of Al2O3 in improving the capacity retention compared with other oxides [15–17].
To assess the influence of surface encapsulation, electrodes with different Al2O3 thicknesses were cycled at an elevated temperature (50°C) in the same potential window of 2.8–4.3 V. Fig. 3(e) presents the rate performance at different current rates of 0.2, 0.1, 0.3, 0.5, 1, 2, and 5C. The conformal coating of Al2O3 appears to have no significance at the lower current rates, as the pristine electrode delivered a higher capacity regardless of the applied current rate. However, an obvious difference is noted at the rate of 5C: the two-cycle ALD coating exhibits a higher capacity than the other coating cycles. Further cyclic stability at this 5C rate is extended for 500 cycles between 2.8 and 4.3 V. Electrodes without the ALD coating exhibit drastic capacity fading and retain only 46.59% after 500 cycles. In contrast, the electrode with a very thin Al2O3 coating (two cycles) has higher capacity retention of 76.33%. An increased thickness of 10 cycles of ALD yields better capacity retention, but at the expense of a low specific capacity, as the thick layer could hinder the diffusion of Li ions at very high charge-discharge rate [34]. The capacity fading of bare electrodes occurs for a variety of reasons: (i) there are deleterious side reactions when the electrodes are charged to a higher operating voltage of 4.3 V, at which most of the non-aqueous electrolytes are easily oxidized [38], (ii) the presence of highly reactive Ni4+ at the end of the charge could fasten the electrolyte decomposition [39], (iii) the migration of transition Ni4+ metal ions from octahedral 3a sites to Li sites (octahedral 3b) at delithiated state causes irreversible structural change to the rock salt and spinal phase, hindering Li+ ion migration [40], (iv) the fluorinated anion species in the electrolyte corrodes the interfacial layer, resulting in decomposition products such as LiF, LixPFy, and LixPOyFz, along with other gaseous products such as CO2 and alkanes [41a], (v) the continuous development of microstrain in the unmodified sample results in loss of crystallinity and thereby capacity fading [41b,23b], (vi) Over-prolonged cycling can aggravate the dissolution of transition metals, especially Mn. All the aforementioned mechanisms are further aggravated at elevated temperature conditions and cause degradation of the electrodes [42]. Whereas the electrodes with surface modification maintains good structural stability and good capacity retention.
Figs. 4 (a, b) show the electrochemical impedance spectroscopy (EIS) results for NCM811 electrodes coated with different numbers of cycles of Al2O3 coating using ALD. The EIS spectra clearly illustrate the relationship between the impedance and the thickness of the cathode interface coated with Al2O3. Each spectrum has a single semicircle from the high-frequency region to the medium-frequency region, denoting the charge-transfer resistance (Rct) mainly at the electrode-electrolyte interface. [43a, 43b]. To have a quantitative idea of the resistance, the obtained spectra are fitted with an equivalent circuit, as shown in the inset of Fig. 4(a), which has Rs the solution resistance and Rct the charge transfer resistance. The results indicate that NCM811 coated with two cycles of ALD has a lower charge-transfer resistance than the other coating cycles. Thus, an optimized thin ALD coating can act as a protective matrix for the cathode without hindering the movement of ions and electron across the interface. The lower charge-transfer resistance of the coated sample is due to the protective effect of Al2O3 before exposure to O2 and H2O before fabrication [44a]. Impedance is also measured after cycling to assess the stability of the electrodes. The NCM811 electrode tested for 100 cycles at room temperature at 1 C rate was considered. It has similar type of semicircle denoting Rct. The Rct is significantly reduced for all the cells after cycling because of the greater interaction of the electrolyte with the active material after cycling. Nonetheless, the electrode with two-cycle Al2O3 coating exhibits better performance than the others. In general, all surface modified cathodes have lower charge transfer resistance. This is due to the role of surface species resulting from the extensive cycling at higher voltages. The acidic species like HF generated during cycling can act as a catalyst in polymerizing the Alkyl carbonates with the surface species formed during cycling. Also it has a strong influence on the impedance behavior as seen in figure. 4b [44b].
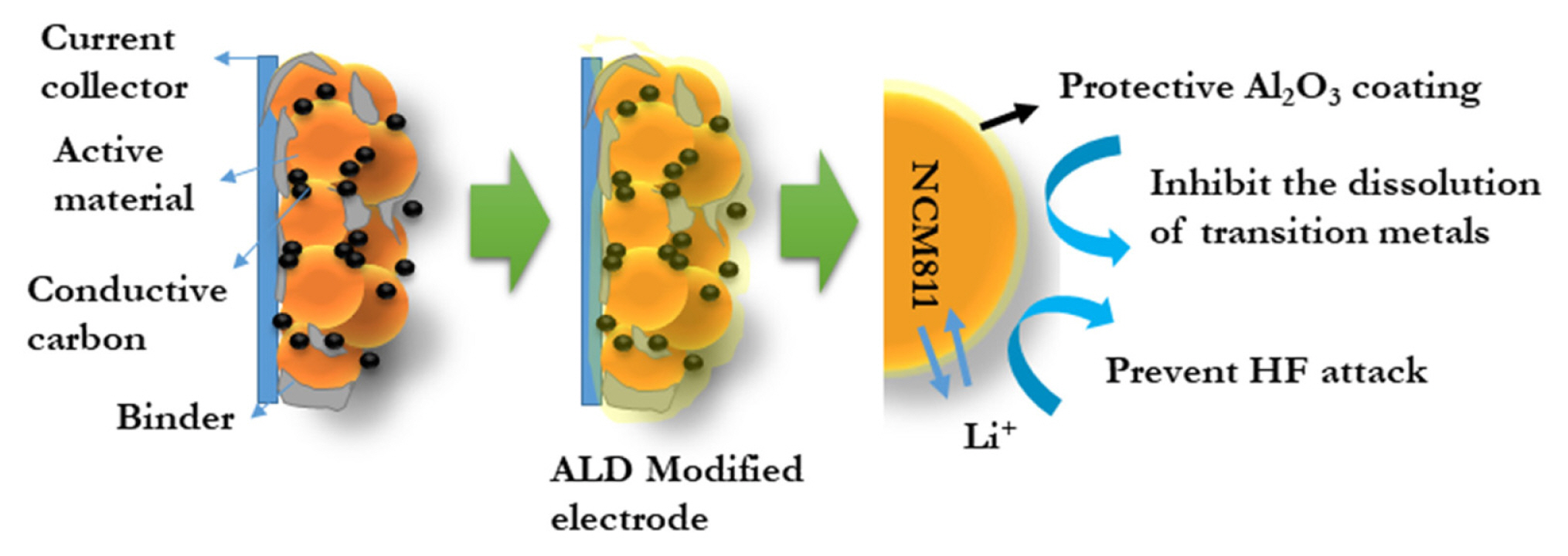
To reveal the robustness of the ALD Al2O3 coating in alleviating the formation of cracks caused by the structural degradation and the volume contraction and expansion during the continuous charge–discharge process, we dismantled the coin cells and analyzed the particle morphology of both the modified and pristine electrodes, and the corresponding FE-SEM image is shown in Fig. 5. The spherical morphology of the NCM811 is well-preserved in the ALD-modified cathode (Fig. 5b). The cycled pristine electrodes have extensive and visible cracks (Fig. 5a). The secondary aggregate particles are highly disintegrated, leading to more penetration of electrolyte to the bulk and accelerate structural degradation along with other surface side reactions resulting in the dissolution of the transition metals in the delithiated state [42]. As analyzed by Wang et al. higher fraction of particles are subjected to severe microstrain, fracture and defects during cycling. All these lead to capacity fading over long cycles at a high temperature [45]. In contrast, the micro-cracks due to the anisotropic volume expansion are not visible even after 500 cycles at a rate of 5C, exhibiting excellent cycle stability, as indicated by the electrochemical performance. This is because the Al2O3 acts as a flexible matrix to accommodate the volume change and prevents the reaction of the electrode with HF generated in the electrolyte, as well as metal dissolution [15]. A schematic depicting the interfacial modification using ALD Al2O3 and its function as a protective covering is provided in Fig. 6.
We successfully modified the interface of the NCM811 cathode by Al2O3 using ALD. High capacity of 190, 199, 188 and 166 mAh g−1 is obtained for pristine, 2, 5 and 10 cycles of ALD coated samples at 0.2C and maintains 145, 158, 151 and 130 mAh g−1 for high current rate of 2C in room temperature. The Al2O3-modified cathode results in 76.33% capacity retention even after 500 cycles (5C) at an elevated temperature, whereas the pristine electrode exhibits only 46.59%. Electrochemical results showed that surface engineering could significantly mitigate the detrimental side reactions along the interface and thereby improve the stability and rate performance of NCM811 under both room-temperature and elevatedtemperature conditions. Post-mortem analysis clearly showed the beneficial effect of surface modification with Al2O3 via ALD. This clearly indicates that the conformal surface modification not only enhances the electrochemical profile but also prevents the structural degradation of cathodes under ambient-temperature and elevated-temperature conditions. This could be a significant step towards realizing high-energy cathode materials for practical applications using conformal surface coating with ALD.
Supplementary Materials
Fig. s1.
TEM elemental mapping of LiNi0.8Co0.1Mn0.1O2 with 50 cycles of ALD Al2O3 modification.
Fig. 1
XRD peaks of LiNi0.8Co0.1Mn0.1O2 obtained from Ecopro (Korea). (b) FE-SEM image of NCM811 with a spherical morphology (c and d). HR-TEM images of 50-cycle ALD Al2O3-modified particles.

Fig. 2
TEM elemental mapping of LiNi0.8Co0.1Mn0.1O2 with two, five, and 10 cycles of ALD Al2O3 modification.

Fig. 3
(a, b) Charge–discharge and differential capacity plot of ALD-modified NCM811 at room temperature (0.2 C rate). (c) Rate capability studies and (d) cycling profiles of Al2O3-modified NCM811 at room temperature at a rate of 1 C. (e) Rate performance of ALD-modified NCM811 electrodes at 50°C. (f) Cycling stability of Al2O3-modified NCM811 electrodes at a rate of 5 C in elevated-temperature conditions.
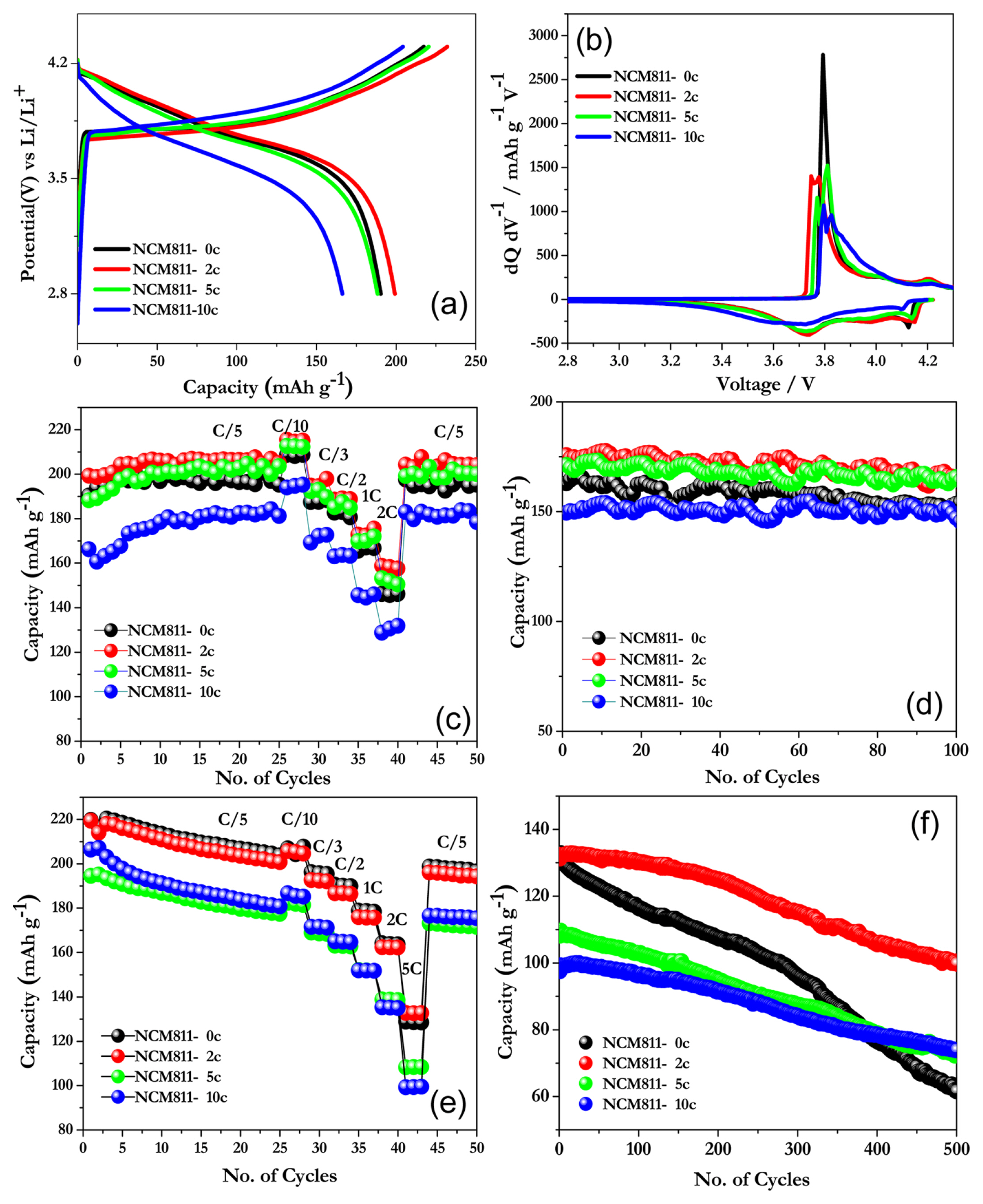
Fig. 4
EIS spectra of ALD Al2O3-modified electrodes (a) before (inset shows the equivalent circuit) and (b) after cycling at a rate of 1 C at room temperature for 100 cycles.

References
[1] D. Andre, SJ. Kim, P. Lamp, SF. Lux, F. Maglia, O. Paschos and B. Stiaszny, J Mater Chem A, 2015, 3, 6709–6732.

[4] M. Latifatu, CY. Bon, KS. Lee, L. Hamenu, Y. Il Kim, YJ. Lee, YM. Lee and JM. Ko, J Electrochem Sci Technol, 2018, 9(4), 330–338.


[6] YK. Sun, Z. Chen, HJ. Noh, DJ. Lee, HG. Jung, Y. Ren, S. Wang, CS. Yoon, ST. Myung and K. Amine, Nat Mater, 2012, 11, 942–947.


[8] D. Mohanty, K. Dahlberg, DM. King, LA. David, AS. Sefat, DL. Wood, C. Daniel, S. Dhar, V. Mahajan, M. Lee and F. Albano, Scientific Reports, 2016, 6, 26532.
[9] SK. Jung, H. Gwon, J. Hong, KY. Park, DH. Seo, H. Kim, J. Hyun, W. Yang and K. Kang, Adv Energy Mater, 2014, 4, 1300787.

[10] LA. Riley, SV. Atta, AS. Cavanagh, Y. Yan, SM. George, P. Liu, AC. Dillon and SH. Lee, J Power Sources, 2011, 196, 3317–3324.

[11] S. Wolff-Goodrich, F. Lin, IM. Markus, D. Nordlund, HL. Xin, M. Asta and MM. Doeff, Phys Chem Chem Phys, 2015, 17, 21778–21781.

[13] J. Zhang, Z. Yang, R. Gao, L. Gu, Z. Hu and X. Liu, ACS Appl Mater Interfaces, 2017, 9, 29794–29803.

[14] W. Zhao, G. Zheng, M. Lin, W. Zhao, D. Li, X. Guan, Y. Ji, GF. Ortiz and Y. Yang, J Power Sources, 2018, 380, 149–157.

[15] F. Schipper, H. Bouzaglo, M. Dixit, EM. Erickson, T. Weigel, M. Talianker, J. Grinblat, L. Burstein, M. Schmidt, J. Lampert, C. Erk, B. Markovsky, DT. Major and D. Aurbach, Adv Energy Mater, 2018, 8, 1701682.

[16] Y. Su, S. Cui, Z. Zhuo, W. Yang, X. Wang and F. Pan, ACS Appl Mater Interfaces, 2015, 7, 25105–25112.

[17] E. Zhecheva, R. Stoyanova, G. Tyuliev, K. Tenchev, M. Mladenov and S. Vassilev, Solid State Sci, 2003, 5, 711–720.

[20] W. Cho, SM. Kim, JH. Song, T. Yim, SG. Woo, KW. Lee, JS. Kim and YJ. Kim, J Power Sources, 2015, 282, 45–50.

[24] HB. Kim, BC. Park, ST. Myung, K. Amine, J. Prakash and YK. Sun, J Power Sources, 2008, 179, 347–350.

[29] YS. Jung, AS. Cavanagh, LA. Riley, SH. Kang, AC. Dillon, MD. Groner, SM. George and SH. Lee, Adv Mater, 2010, 22, 2172–2176.

[30] YS. Jung, AS. Cavanagh, AC. Dillon, MD. Groner, SM. George and S-H. Lee, J Electrochem Soc, 2010, 157, A75–A81.

[31] JW. Kim, JJ. Travis, E. Hu, KW. Nam, SC. Kim, CS. Kang, JH. Woo, XQ. Yang, SM. George, KH. Oh, SJ. Cho and SH. Lee, J Power Sources, 2014, 254, 190–197.

[32] A. Yano, S. Aoyama, M. Shikano, H. Sakaebe, K. Tatsumi and Z. Ogumi, J Electrochem Soc, 2015, 162, A3137–A3144.

[33] W. Liu, X. Le, D. Xiong, Y. Hao, J. Li, H. Kou, B. Yan, D. Li, S. Lu, A. Koo, K. Adair and X. Sun, Nano Energy, 2018, 44, 111–120.

[34] ST. Myung, K. Izumi, S. Komaba, YK. Sun, H. Yashiro and N. Kumagai, Chem Mater, 2005, 17, 3695–3704.

[37] W. Lu, L. Liang, X. Sun, X. Sun, C. Wu, L. Hou, J. Sun and C. Yuan, Nanomaterials, 2017, 7, 325.

[38] G. Cherkashinin, M. Motzko, N. Schulz, T. Spath and W. Jaegermann, Chem Mater, 2015, 27, 2875–2887.

[41a] R. Younesi, AS. Christiansen, R. Scipioni, DT. Ngo, SB. Simonsen, K. Edstrom, J. Hjelm and P. Norby, J Electrochem Soc, 2015, 162, A1289–A1296.

[42] S. Watanabe, M. Kinoshita, T. Hosokawa, K. Morigaki and K. Nakura, Journal of Power Sources, 2014, 258, 210–217.

[43a] HV. Ramasamy, K. Kaliyappan, R. Thangavel, V. Aravindan, K. Kang, DU. Kim, Y. Park, YS. Sun and X. Lee, J Mater Chem A, 2017, 5, 8408.

[43b] HV. Ramasamy, K. Kaliyappan, R. Thangavel, WM. Seong, K. Kang, Z. Chen and YS. Lee, J Phys Chem Lett, 2017, 8, 5021–5030.

[44a] R. Zhao, J. Liang, J. Huang, R. Zeng, J. Zhang, H. Chen and G. Shi, J Alloy and Comp, 2017, 724, 1109–1116.

[44b] D. Aurbach, K. Gamolsky, B. Markovsky, G. Salitra, Y. Gofer, U. Heider, R. Oesten and M. Schmidt, J Electrochem Soc, 2000, 147(4), 1322–1331.

- TOOLS
-
METRICS

-
- 24 Web of Science
- 0 Crossref
- Scopus
- 9,494 View
- 178 Download
- Related articles in J. Electrochem. Sci. Technol