1. Introduction
The demand of high energy density for lithium-ion batteries (LIBs) rapidly grows because of superior high energy density devices. Beyond lithium-ion batteries, lithium metal batteries (LMBs) are thought of as the next generation of rechargeable batteries due to the extremely high theoretical specific capacity (3860 mAh g−1, 2062mAh cm−3) and the lowest redox potential (−3.04 V vs. standard hydrogen electrode) [1–3].
However, LMBs has severe problems due to (1) uncontrollable lithium dendrite formation, result in penetration of the separator, causing short circuit, (2) large volumetric and morphological changes during charging process, (3) continuous reactions between lithium metal and electrolyte resulting from the crack of solid electrolyte interphase (SEI) layers on the lithium metal surface [4,5]. These problems result in deterioration of cycle life and safety risk.
Several strategies have been explored such as varying the electrolyte (lithium salt, solvent (carbonate-, ether-) and functional additives) to form a stable SEI layer [6,7], lithium protective layers to suppress lithium dendrite growth [8–10], the separator design [11], and the external pressure [12–14]. Among these challenge issues, the development of new electrolytes and protective coating layers needs more cost and process. However, the external pressure for pouch-type batteries is effective method because commercial lithium-ion battery cells are assembled by an external case which can apply low level of the pressure. In case of LMBs, high constant pressure is needed to supress lithium dendrite growth.
Therefore, we presented the effect of external pressure of the lithium metal pouch cell with large area to suppress lithium dendrite and uniformly form SEI layers. It is effective method to prevent the swelling of the lithium metal battery.
2. Experimental
LiNi0.83Co0.11Mn0.06O2 (NCM83) was prepared from Cosmo AM&T Co. For preparation of the cathode electrode, a slurry containing 95 wt% NCM83, 2.5 wt% conductive carbon (Denka black), and 2.5 wt% polyvinylidene fluoride (PVdF) binder in N-methyl-2-pyrrolidone (NMP) solvent was coated onto both side of Al foil (15 μm thickness) by using a coating machine. After coating, the cathode electrode was adjusted to control the loading level at 15 mg cm−2 and the electrode density at 3.25 g cm−3. The prepared cathode electrode was punched into rectangular pieces (50 mm × 80 mm) for the pouch type cell. The lithium metal foil (100 μm thickness, Honzo, Japan) was used and attached onto one side of 11 μm thickness Cu foil by the roll press machine. The prepared lithium anode was punched into rectangular pieces (52 mm × 82 mm). The polyethylene (PE, 20 μm thickness) was used as the separator. 3M Lithium bis(fluorosulfonyl)imide (LiFSI) DME + 1 wt% Lithium difluoro(bisoxalato)phosphate (LiD-FBP) + 3% LiNO3 (Soulbrain Co.) was used as the electrolyte because ether-based electrolytes are relatively stable at the lithium anode compared to carbonate-based electrolytes and high salt concentration improves the oxidation stability of the ether-based electrolytes [15–18]. The LiDFBP was used to form solid electrolyte interphase with LiF for Li metal anodes to prevent the lithium dendrite and the LiNO3 was used to form Li3N at the cathode-electrolyte interphase for enhancing lithium ion conductivity of Ni-rich cathodes [17,19,20].
The pouch cell composed of one of the cathode, the two of anode, the PE separator and the electrolyte. The pouch cell was prepared as illustrated in Fig. 1(a) and specific electrode design in Table S1. Al external tabs for cathodes and Ni external tabs for anodes was connected by ultrasonic welding. The stacked electrode was packed by Al laminated pouch and vacuum sealing with electrolyte injection (E/C ratio = 5). The pouch cell was pressed by a press jig with 4 different external pressure conditions such as 0.6, 0.7, 0.8 psi and without the external pressure (W/O pressure) which is shown in Fig. 1(b). The pouch cells were pressed by the press jig with a bolt at each corner. The pressure is provided by four bolts was tightened by the digital torque wrench with the same force. The force value was converted to pressure value. The pouch cell capacity has 208 mAh (in a potential window between 3.0 and 4.2 V) capacity. All procedure was performed in the dry room (relative humidity (RH) 0.1%, 20°C). The cycling performance was tested under the voltage range of 3.0–4.2 V at 1 C-rate (1 C = 185 mAh g−1) using an electrochemical equipment (Maccor, Series 4000) at 25°C. The discharge rate capability was carried out at a various discharge C-rates (1 C, 2 C, and 3 C) with fixed charge C-rate (1 C).
Electrochemical impedance spectroscopy (EIS) measurements were conducted in the frequency range of 1 MHz–10 mHz with 5 mV amplitude using a VSP300 impedance analyzer. The morphologies were measured via field emission scanning electron microscopy (FE-SEM, S-4800, HITACHI).
3. Results and Discussion
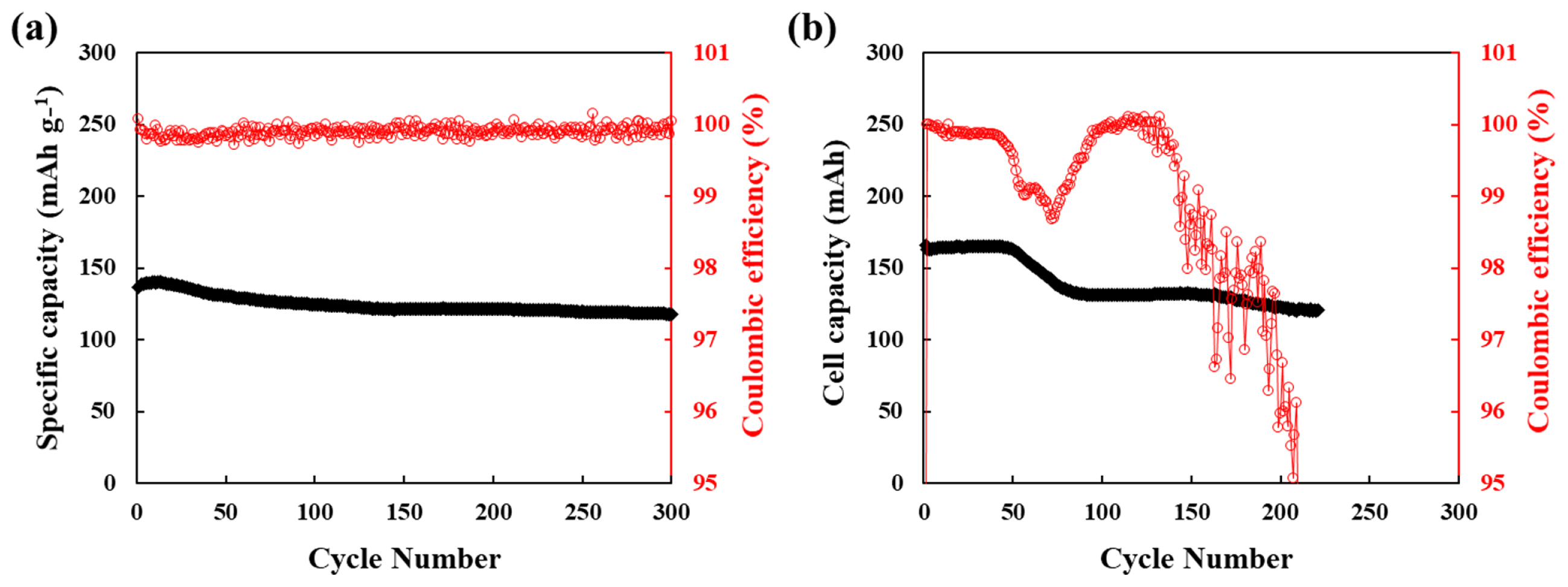
The comparison of cycling performance of the coin type cell and the pouch type cell with external pressure by binder clip is shown in Fig. 2. In case of coin type cell, the cycling retention was better than pouch type cell. The pouch cell type cell showed dramatically decrease after 50 cycles and the coulombic efficiency was a highly unstable. It is related with the uncontrolled lithium dendrite growth [21,22]. The lithium metal is provided proper constant pressure in coin type cell, however, there was lack of proper pressure to form stable formation of active lithium metal in the pouch type cell. To meet practical lithium metal batteries, it seems that a proper external pressure is essential.
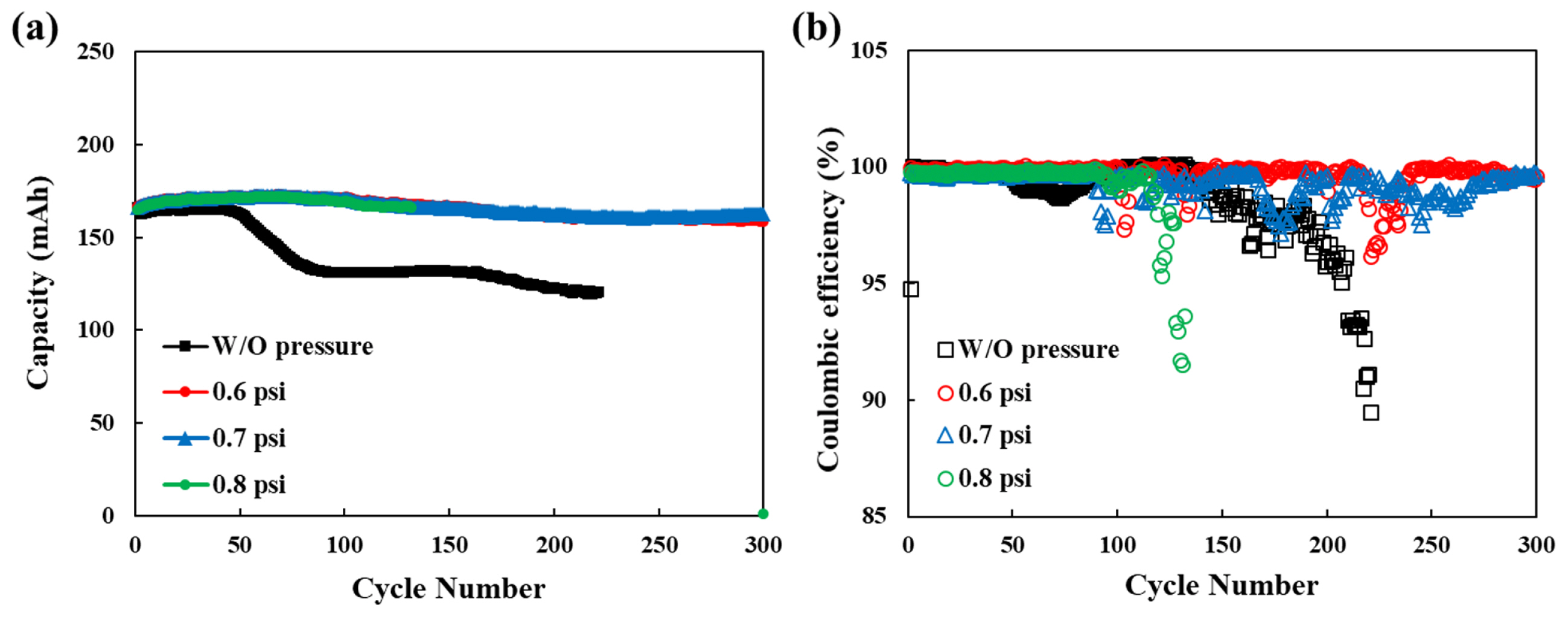
The effect of external pressure change is investigated by electrochemical performances in Fig. 3. The cycling performance was tested at 1 C rate with different external pressures. The coulombic efficiency exhibited fluctuations during cycling test. The small drop on coulombic efficiency is ascribed to internal soft short and it recovers soon. However, the sudden drop on coulombic efficiency under 95% do not recover again and the tests were stopped due to the safety problem. The discharge capacity of the pouch cell without pressure rapidly decreased from 50 cycles, whereas the pouch cells with pressure stably retained the discharge capacity. The rapidly decrease of discharge capacity without pressure is associated with SEI on lithium surface. The lithium dendrite can strip from lithium metal and it reveal the bare lithium. Therefore, the new electrochemical reaction between the lithium anode and the electrolyte generate SEI layers. This result decreases the discharge capacity and the coulombic efficiency [10,13,23].
At 0.8 psi, the columbic efficiency suddenly dropped under 95%, so the test was stopped. In our experience for Li-metal battery, when the columbic efficiency dramatically dropped, the cell suddenly burst (Fig. S1). This result can be ascribed to the high external pressure because the soft lithium metal can penetrate the PE separator due to the high pressure.
Therefore, the distance between the lithium anode and the cathode became near, and the lithium dendrite locally grow in the separator. However, the pouch cells with proper pressure (0.6 and 0.7 psi) kept the cyclability by 300 cycles at 1 C-rate. The capacity retention of the pouch cells at 0.6 and 0.7 psi exhibits 91.9% and 94.2%, respectively after 300 cycles.
The EIS was measured after the cycling test to further elucidate the resistance of the cell in Fig. 4. The acquired EIS curve consists of two semicircles. The initial point on the x axis is related to the electrolyte resistance (Rs), the first semicircle stands for the solid electrolyte interface (RSEI), and the second semicircle corresponds to the charge transfer resistance (Rct) [19]. The cell without the external pressure showed the totally higher resistance than the cells with the external pressure. The Rs especially increased compared to the cells with the pressure. It means the decomposition of the electrolytes due to the continuous reaction with lithium anode. The cells with the external pressure have similar the Rs and RSEI at 0.6 and 0.7 psi. However, the cell with a pressure of 0.7 psi showed the lower Rct than the cell with a pressure of 0.6 psi, resulting in the higher electrochemical performance.
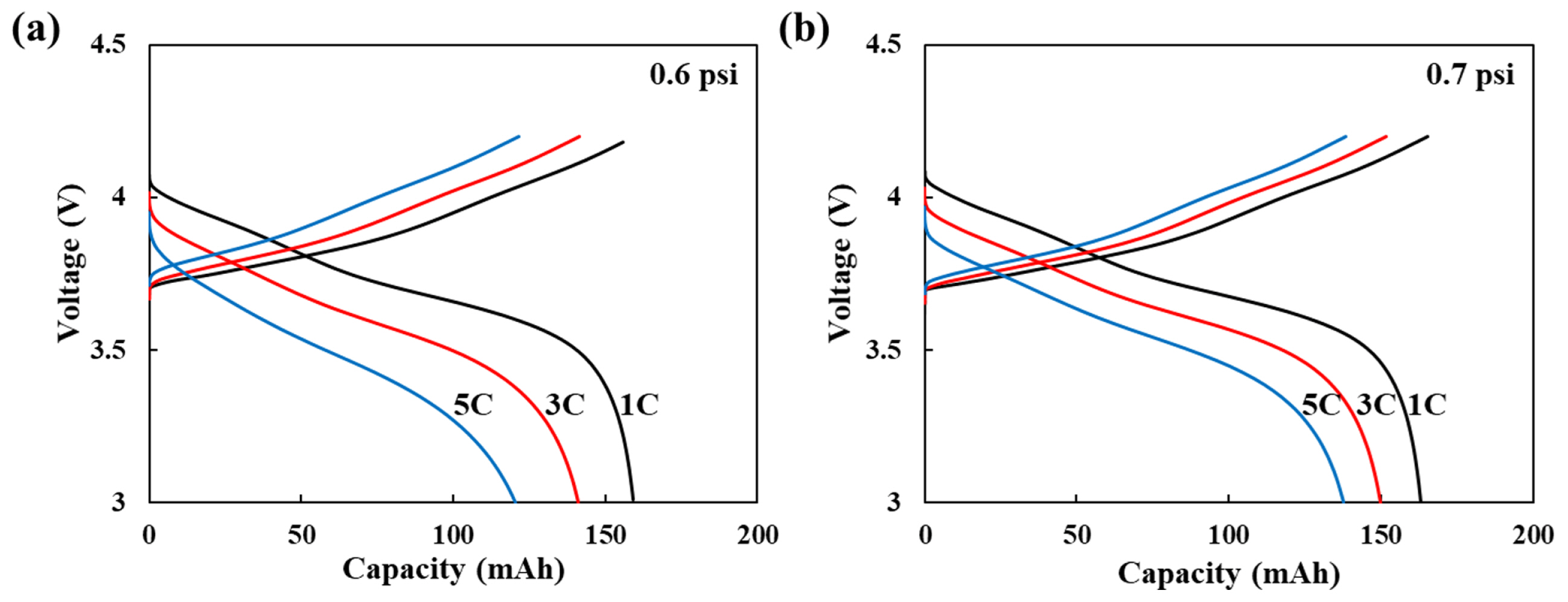
Moreover, their rate capability was also tested to distinguish the electrochemical performance in Fig. 5. The rate capability was estimated after 300 cycling test samples. At 0.6 psi, the discharge retention of 3 C and 5 C was 88.4% and 75.5%, respectively, compared to 1 C rate. At 0.7 psi, the discharge retention of 3 C and 5 C was 92.0% and 84.4%, respectively, compared to 1 C rate. The cyclability is associated with the resistance of the cell.
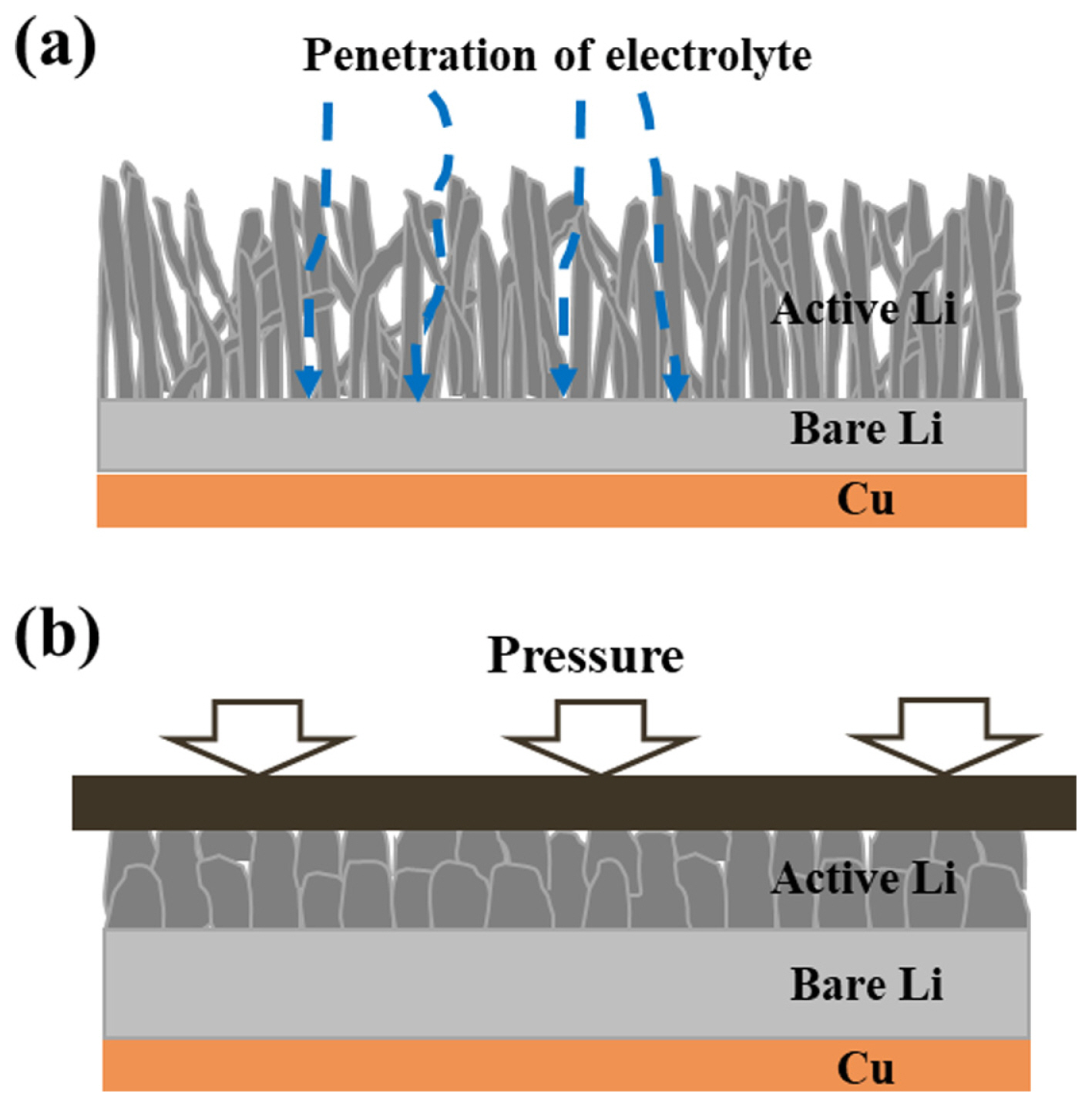
The major reason of the degradation in lithium metal batteries is uncontrolled growth of the lithium metal like mossy/dendritic lithium. It generates the penetration of the separator, expansion of the active lithium metal resulting in the cell swelling, decomposition of the electrolytes due to side effects with the lithium anode, and the much consumption of the bare lithium metal [24]. As the results, the proper external pressure enhances the electrochemical performance and the stability of the lithium metal cell during cycling. It is associated with the suppression of the growth of mossy/dendritic lithium metal.
In order to further elucidate the effect of the external pressure, the lithium anode was confirmed after cycling test in Fig. 6. When disassembling, the active lithium metal of the cell without the external pressure was easily separated from the lithium anode and it showed as powder morphology (Fig. 6(a)). In contrast, the active lithium metal with the external pressure at 0.7 psi strongly attached on the lithium anode (Fig. 6(c)). This result can be confirmed from FESEM images (Fig. 6(b)). Without the external pressure, the thickness of the lithium metal expanded by about 400%. Moreover, the formation of lithium growth becomes porous. On the other hand, with the external pressure, the lithium metal only grew by about 70%, and the lithium formed firm and dense. Even bare lithium remained with a thickness of 55 μm, about half of the first. (Fig. 6(d)). The porous lithium metal accelerates the side reactions between the lithium metal and the electrolytes. The reaction continuously occurs and the electrolytes easily react with bare lithium through by porous lithium metal, and the bare lithium metal and the electrolyte are considerably consumed. However, the lithium metal formed dense by the external pressure is suppress the penetration of the electrolyte into the bare lithium metal, and the unnecessary reaction is reduced. The growth of the lithium anode during cycling is represented in Fig. 7. Thus, the proper pressure for pouch cells is necessary technology for lithium metal batteries to perform long cycling performance and safety. Because the suppression of porous growth of lithium metal prevents the unnecessary reaction and cell swelling, the result can reduce the resistance of the cell and safety problems.
The proper external pressure considerably helps long stable cycle because the pressure prevents the vertical and porous growth of lithium dendrite and strongly fixes dead lithium particles.
4. Conclusions
In summary, the proper external pressure enhances the cyclability of lithium metal batteries. At a pressure of 0.7 psi, the lithium metal cell showed the best capacity retention ratio of 94.2% after 300 cycles. The external pressure prevents the porous lithium metal and reduces the unnecessary reaction between the electrolyte and the lithium metal. Finally, we can conclude that the external pressure equipment can be applied as one of the ways to improve the electrochemical performance of lithium metal batteries in a practical pouch cell.