|
 |
- Search
| J. Electrochem. Sci. Technol > Volume 15(1); 2024 > Article |
|
Abstract
Despite the important role of manganese (Mn) in cobalt-free, Ni-rich cathode materials, existing reports on the effects of Mn as a substitute for cobalt are not consistent. In this work, we analyzed the performance of cathodes comprised of Li(Ni1–xMnx)O2 (LNMO). Both beneficial and detrimental results occurred as a result of the Mn substitution. We found that a complex interplay of effects (Li/Ni mixing driven by magnetic frustration, grain growth suppression, and retarded lithium insertion/extraction kinetics) influenced the performance and was intimately related to calcination temperature. This indicates the importance of establishing an optimal reaction temperature for the development of high-performance LNMO.
LiNiO2 (LNO) is a potentially attractive electrode material for lithium-ion batteries due to its cost-effectiveness and high discharge capacity (~240 mAh g−1) [1]. Upon cycling, however, it experiences serious structural degradation due to the generation of highly reactive Ni4+ species and the abrupt anisotropic volume change in the deeply charged state, leading to rapid capacity decline [2,3]. While Co- and/or Mn-incorporated Ni-rich cathode materials (e.g., LiNix-CoyMnzO2 (NCM) and LiNixCoyAlzO2 (NCA)) partly addressed this issue of capacity fading, the toxicity and scarcity of Co have prompted the elimination of Co from cathode materials in recent years [4,5]. Thus, tremendous efforts have been made to develop cobalt-free, Ni-rich cathode materials, such as Li(Ni1–xMnx)O2 (LNMO), that can deliver high energy density while preserving capacity retention over many cycles [6,7]. Despite successful demonstrations of LNMO as an alternative to NCM and NCA [8–10], there is a wide variation in the performance of LNMO as reported in the literature, which we ascribe to suboptimal synthesis conditions. Given the substantial impact of synthesis conditions on the performance of Ni-rich cathode materials [11–13], it is important to explore their effects and to establish optimal synthesis conditions. To this end, we investigated the influence of lithiation temperature on the electrochemical performance of LNMO. Our research revealed that the performance was temperature-dependent. This temperature dependency varied substantially with Mn composition. The underlying reasons are elucidated in this work.
We prepared Ni1-xMnx(OH)2 (x = 0, 0.05, 0.1, 0.15, and 0.2) precursors according to the traditional co-precipitation method [14]. There were no noticeable differences between the precursors in terms of crystal structure and morphology (Fig. S1 and S2), demonstrating the successful production of precursors. The precursors were thoroughly mixed with LiOH·H2O, and each mixture was annealed under oxygen gas flow: first at 500°C for 5 h and then at one of two temperatures (650°C or 800°C) for 10 h. Spherical particles with diameters 8–10 μm were formed regardless of temperature (Fig. S3), revealing that the temperature variations did not affect the morphology of the secondary particle. The resulting products will hereafter be abbreviated as LNMO(A)-(B), where (A) is the atomic percentage of Mn content and (B) is the calcined temperature; for example, LiNi0.95Mn0.05O2 synthesized at 650°C is labeled as LNMO5-650. Slurries composed of the active material, Super P, and polyvinylidene fluoride were mixed in a weight ratio of 85:7.5:7.5 and then were coated onto Al foil and dried at 100°C for 1 h in a vacuum oven. The resulting films were roll-pressed and further dried in the vacuum oven for 12 h. Each film was assembled into CR 2032-type half-cells in an Ar-filled glove box. We used Li foil as the reference electrode, Celgard 2320 as the separator, and 1.13 M LiPF6 solution in an ethylene carbonate/dimethyl carbonate (3:7 in volume) as the electrolyte. The surface morphologies and crystal phase information of all precursors and corresponding products were obtained using scanning electron microscopy (SEM; Hitachi S-4800) and X-ray diffraction (XRD; Rigaku D/Max-2500/PC). The stoichiometric compositions of the samples were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES) using the Varian 715 ES instrument. The electrochemical performance of the half-cells was evaluated using battery cyclers (WBCS3000, Wonatech) at 1C (195 mAh g−1) within the potential range of 2.7–4.3 V versus Li+/Li at 25°C.
The role of substituted Mn is crucial in Ni-rich cathode materials, particularly in Co-free systems, because Mn is the only element (except Al) that can be homogeneously introduced into Ni(OH)2 at greater than 5% [15]. The substituted Mn in LNMO is beneficial because it can not only suppress irreversible phase transitions but also decrease electrode reactivity with electrolyte on the surface, securing greater thermal stability of LNO [16]. Despite these benefits, research on the Mn substitution effects has reported inconsistent results. We attribute this to unoptimized reaction conditions, given our previous report [11]. Performance comparisons between LNMOs with varying Mn content prepared at two extreme temperatures (650°C and 800°C) demonstrate this discrepancy (Fig. 1). The overall measured ICP results are appropriate with the desired Mn contents at these two temperatures (Table S1 in the Supporting Information). From Fig. 1, in terms of initial capacity and cycling stability, 5% Mn-substituted LNO (LNMO5) shows noticeably different performance at different temperatures. While LNMO5-650 showed reasonably stable capacity retention over 100 cycles (Fig. 1A), LNMO5-800 exhibited a significant capacity decline (Fig. 1B). However, the initial capacity of LNMO5-800 was greater than that of LNMO5-650. This observation implies that careful temperature control during the calcination of LNMO is critical to fully utilize the advantages of Mn substitution. The current understanding of the influence of substituted Mn on the battery performance of LNMO is inadequate. The overall trend observed in Fig. 1 is that, at the lower calcination temperature, the capacity retention is relatively good but occurs at the expense of capacity as the Mn content gradually increases. However, when LNMO was synthesized at the higher temperature, the initial capacity was significantly increased until Mn incorporation reached 15%, although 20% Mn substitution led to only a slight increase compared to its counterpart prepared at 650°C. For cycling stability, the increased Mn content gradually improved capacity retention until the substituted Mn content exceeded 15%, at which a noticeable increase was observed. These observations illustrate the confusion regarding the factors that govern battery performance. To this end, we explored a structure-performance relationship via in-depth crystallographic analysis.
Fig. 2 exhibits the XRD patterns of all prepared LNMO samples, along with Rietveld refinement results (see Table 1 for the detailed parameters). Regardless of calcination temperature, a noticeable tendency in the XRD patterns was decreased (003)/ (104) peak ratios with increased Mn content. This tendency was also manifested with gradually increased lattice parameters of the c-axis (Table 1), which is due to the strong interaction between the magnetic spins of Mn and Ni ions, known as magnetic frustration [17,18]. The structural instability driven by the magnetic frustration can be relieved by Li/Ni exchange, which was consistent with increased Ni2+ content in the Li slabs (Table 1). Excess liberation of Ni2+ ions at Li sites is detrimental to Li+ migration, which eventually leads to capacity loss. In our investigation, a careful examination of Ni2+ content in the Li slabs revealed a strong correlation between the degree of Li/Ni disorder and the initial capacity. The gradual capacity decline observed in LNMOs synthesized at 650°C (213, 196, 172, and 170 mAh g−1) is consistent with the changes in Ni2+ content of the Li slabs (4.2, 8.1, 11.3, and 16.7%). Also, the high initial capacity observed in LNMO5-800, LNMO10-800, and LNMO15-800 is intimately associated with the relatively small amount of Ni2+ content in the Li slabs (less than 7%). The sudden decrease in capacity in LNMO20-800 can be ascribed to the nearly doubled Ni2+ content (11.7%). These results indicate that high-temperature calcination can effectively mitigate Li/Ni disorder induced by magnetic frustration. However, we found that this beneficial effect on the integrity of the layered structure was decreased greatly if the Mn content was greater than a critical amount (e.g., 20% Mn in the case of calcination at 800°C).
Another effect of the introduction of Mn into LNO was found in grain growth kinetics. The SEM images in Fig. 3 show a dramatic difference in primary particle sizes between LNMO calcined at 650°C and that calcined at 800°C. This suggests that the substituted Mn acted as a grain growth inhibiter. This effect of substituted Mn on crystal growth is illustrated in the LNMO samples prepared at 800°C. When 5% Mn was introduced to LNO (i.e., LNMO5-800), the grain size was 126.7 nm. However, the grain size of LNMO20-800 was as small as 35.6 nm, confirming that Mn can substantially suppress grain growth (See Table 1 for the grain sizes of all other samples calculated using the Scherrer equation.). In the LNMOs synthesized at 650°C, a similar trend in grain size as a function of Mn content was observed. However, because the grain size of LNMO5-650 was merely 30.6 nm, the grain growth was significantly inhibited (almost 4 times smaller than that of LNMO5-800). This implies that the temperature of 650°C was not sufficient to promote grain growth during the lithiation reaction.
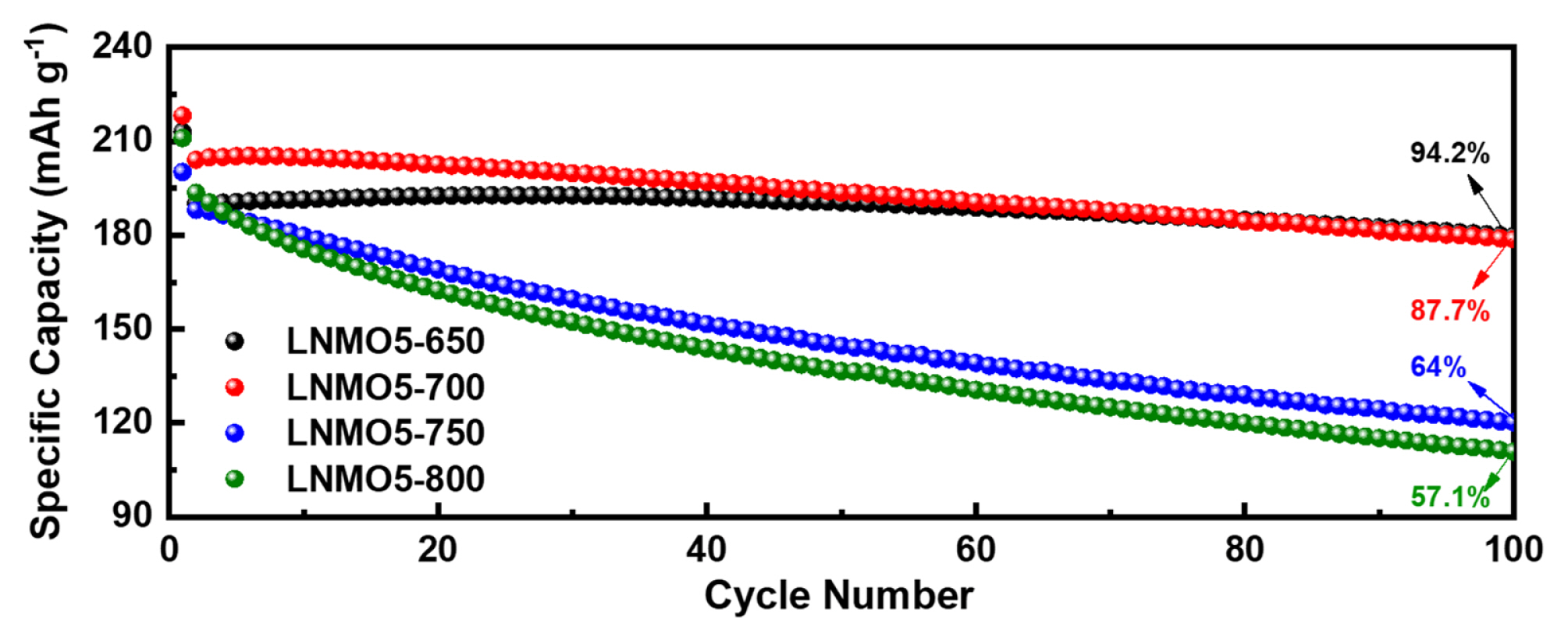
Grain size is an important factor in cathode performance [19,20]. Small grain sizes can not only facilitate lithium diffusion by shortening Li diffusion length but also alleviate mechanical strain accumulated during the H2–H3 phase transition to improve capacity retention over cycles. This was the case in our study, where the capacity retention of the LNMOs prepared at 650°C was superior regardless of Mn content. However, the capacity retention of LNMOs at 800°C was strongly dependent on the amount of substituted Mn (Fig. 1). LNMOs with grain size less than 58.6 nm (LNMO15-800 and LNMO20-800) maintained decent capacity retention capability. This indicates a strong correlation between grain size and the structural robustness of the LNMO samples. In addition, differential capacity (dQ dV−1) plots (Fig. 4 and 5) revealed that the potential at which the H2–H3 phase transition occurred (typically 4.2~4.5 V) gradually increased as Mn content increased. This effect can be beneficial in terms of sustaining good cycling performance, as witnessed in the present study, but detrimental in terms of capacity loss (i.e., no capacity contribution from the H2–H3 phase transition). The degree of this potential shift (H2–H3 phase transition) depends on calcination temperature (as can be observed in the difference between LNMO15-650 and LNMO15-800), implying that careful calcination temperature control is crucial for the synthesis of high-performance Co-free, Ni-rich cathode materials. As a demonstration of the importance of temperature control, we synthesized 5% Mn-substituted LNMO at four different temperatures (Fig. S4 and Table S2 for the Rietveld refinement results), and the resulting battery performance is presented in Fig. 6. While fine temperature tuning (presumably in the range between 650°C and 700°C) is required, the best compromise between the beneficial and detrimental effects of Mn was achieved at 700°C.
Magnetic frustration, grain growth suppression, and retarded lithium insertion/extraction kinetics are major effects of substituted Mn in LNMO. The present study shows that these effects are competing factors in terms of battery performance and interact complexly. In addition, the influence of Mn is strongly dependent on the calcination temperature. High-temperature calcination for the synthesis of LNMO can be beneficial for enhancing structural order and suppressing the reduction of lithium diffusion kinetics (to decrease capacity loss). However, these beneficial effects are counterbalanced by facilitated grain growth (which can deteriorate capacity retention). We concluded that precisely controlling the temperature and minimizing temperature fluctuations are of great importance in the utilization of Mn substitution. Therfore, additional research on calcination conditions for Nirich cathode materials of varying compositions is further needed. This type of future research would build upon the knowledge gained in this study and advance our understanding of the Mn substitution effect.
Acknowledgments
This research was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2022R1A2C2006654) and by the Ministry of Education (NRF-2018R1A6A1A03024231). It was also supported by Brain Pool Program funded by the Ministry of Science and ICT through the NRF (NRF-2020H1D3A1A02081147). We appreciate the financial support from NeonTech Co., Ltd.
Supporting Information
Supporting Information is available at https://doi.org/10.33961/jecst.2023.00787
Fig. 1
Comparative cyclability of the half-cells made of LiNi1-xMnxO2 (x = 0, 0.05, 0.1, 0.15, and 0.2) cathode materials prepared at (A) 650°C and (B) 800°C.

Fig. 2
XRD Rietveld refinement results of (A) LNMO5-650, (B) LNMO10-650, (C) LNMO15-650, (D) LNMO20-650, (E) LNMO5-800, (F) LNMO10-800, (G) LNMO15-800, and (H) LNMO20-800.

Fig. 3
SEM images of (A) LNMO5-650, (B) LNMO10-650, (C) LNMO15-650, (D) LNMO20-650, (E) LNMO5-800, (F) LNMO10-800, (G) LNMO15-800, and (H) LNMO20-800.
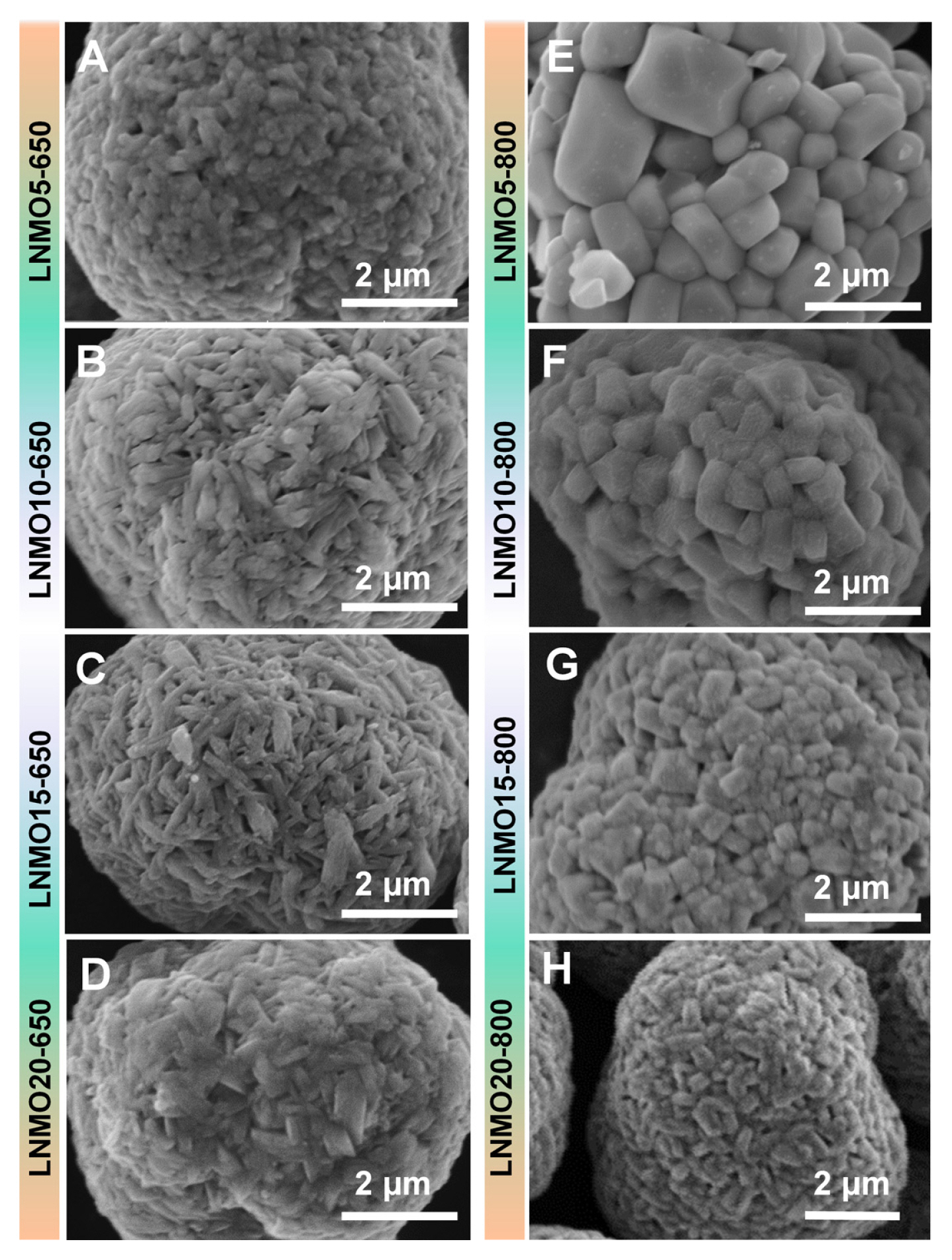
Fig. 4
Charge/discharge profiles and corresponding dQ dV−1 curves of LiNi1-xMnxO2 electrodes calcined at 650°C: (A) LNMO5-650, (B) LNMO10-650, (C) LNMO15-650, and (D) LNMO20-650.
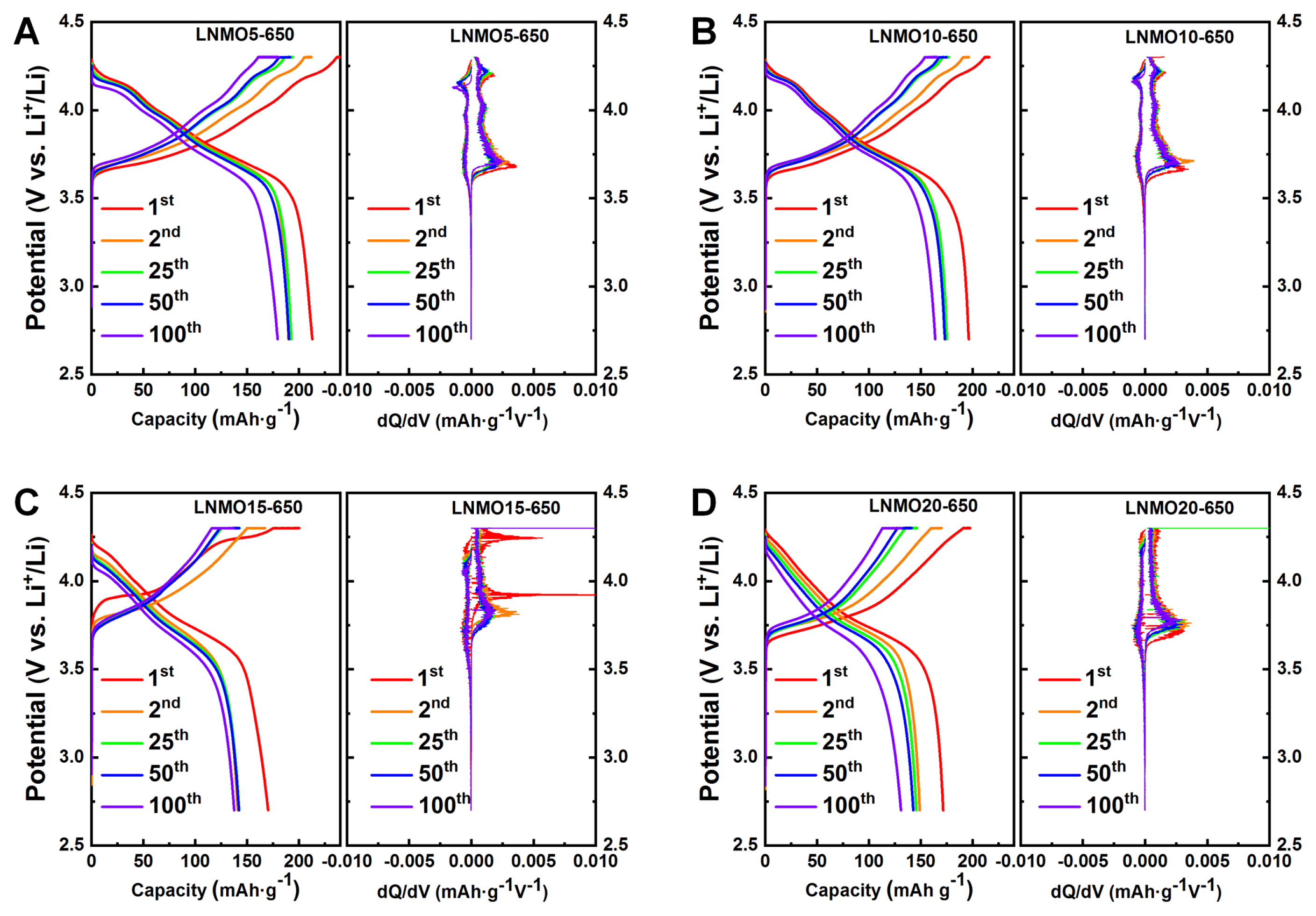
Fig. 5
Charge/discharge profiles and corresponding dQ dV−1 curves of LiNi1-xMnxO2 electrodes calcined at 800°C: (A) LNMO5-800, (B) LNMO10-800, (C) LNMO15-800, and (D) LNMO20-800.
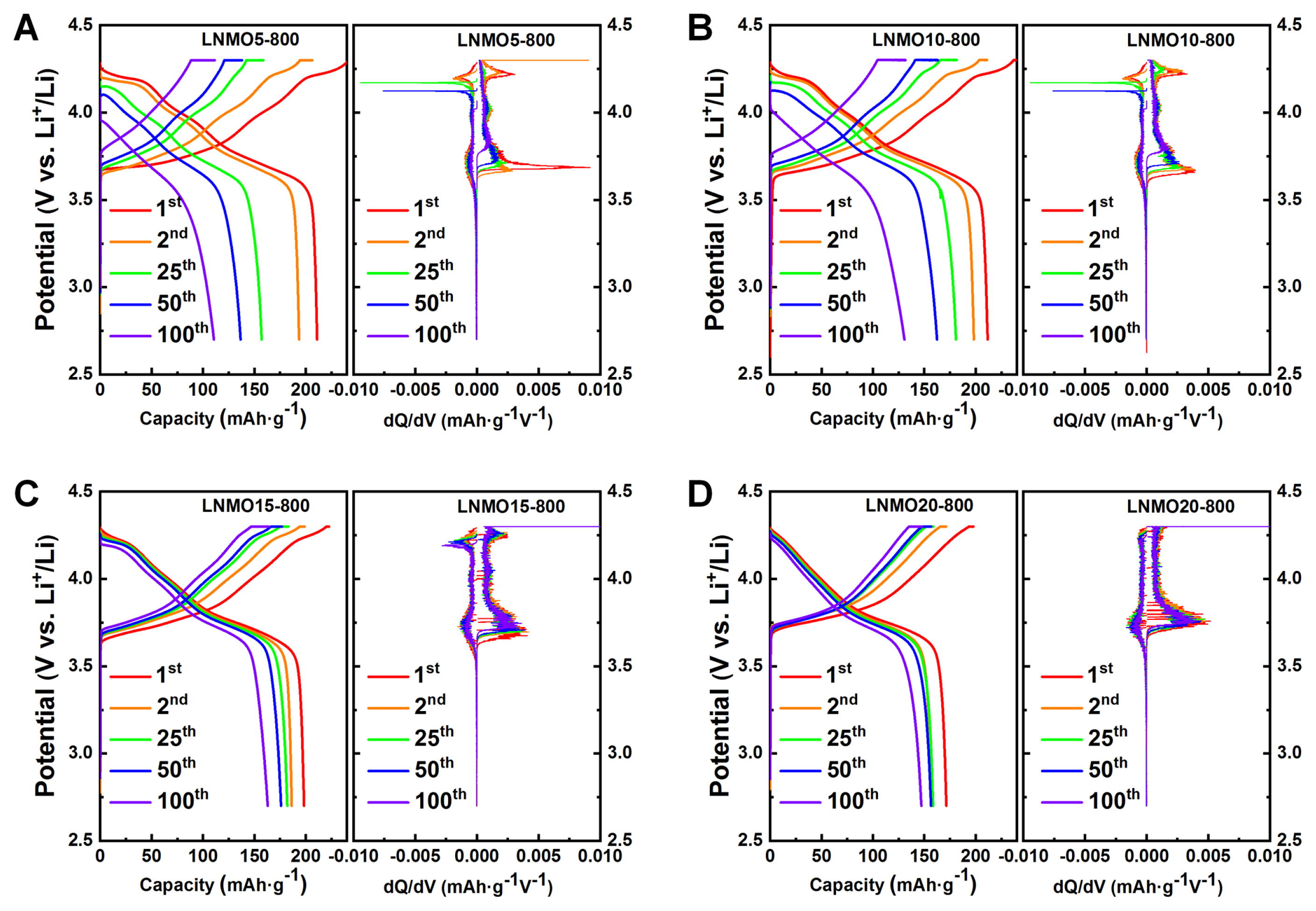
Table 1
Rietveld refinement results of LiNi1-xMnxO2 produced at 650 and 800°C
References
[1] S.-T. Myung, F. Maglia, K.-J. Park, C. S. Yoon, P. Lamp, S.-J. Kim and Y.-K. Sun, ACS Energy Lett., 2017, 2(1), 196–223.

[2] R. Pan, E. Jo, Z. Cui and A. Manthiram, Adv. Funct. Mater., 2023, 33(10), 2211461.
[3] M. Bianchini, M. Roca-Ayats, P. Hartmann, T. Brezesinski and J. Janek, Angew. Chem. Int. Ed., 2019, 58(11), 10434–10458.


[4] G. T. Park, H. H. Sun, T.-C. Noh, F. Maglia, S.-J. Kim, P. Lamp and Y.-K. Sun, Adv. Energy Mater., 2022, 12(48), 2202719.
[5] H. Li, M. Cormier, N. Zhang, J. Inglis, J. Li and J. R. Dahn, J. Electrochem. Soc., 2019, 166(4), A429–A439.

[7] H. Li, L. Wang, Y. Song, Y. Wu, H. Zhang, A. Du and X. He, Small, 2023, 19(32), 2302208.
[8] T. Liu, L. Yu, J. Liu, J. Lu, X. Bi, A. Dai, M. Li, M. Li, Z. Hu, L. Ma, D. Luo, J. Zheng, T. Wu, Y. Ren, J. Wen, F. Pan and K. Amine, Nat. Energy, 2021, 6, 277–286.


[9] Y.-K. Sun, D.-J. Lee, Y. J. Lee, Z. Chen and S.-T. Myung, ACS Appl. Mater. Interfaces, 2013, 5(21), 11434–11440.

[10] L. Yu, H. Zhao, J. Sun, Q. Han, J. Zhu and J. Lu, Adv. Funct. Mater., 2022, 32(40), 2204931.
[12] P. Kurzhals, F. Riewald, M. Bianchini, H. Sommer, H. A. Gasteiger and J. Janek, J. Electrochem. Soc., 2021, 168, 110518.


[15] Z. Cui, Z. Guo and A. Manthiram, Adv. Energy Mater., 2023, 13(12), 2203853.
[16] W. Li, S. Lee and A. Manthiram, Adv. Mater., 2020, 32(33), 2002718.
[17] Y. Xiao, T. Liu, J. Liu, L. He, J. Chen, J. Zhang, P. Luo, H. Lu, R. Wang, W. Zhu, Z. Hu, G. Teng, C. Xin, J. Zheng, T. Liang, F. Wang, Y. Chen, Q. Huang, F. Pan and H. Chen, Nano Energy, 2018, 49, 77–85.

[18] J. Zheng, G. Teng, C. Xin, Z. Zhuo, J. Liu, Q. Li, Z. Hu, M. Xu, S. Yan, W. Yang and F. Pan, J. Phys. Chem. Lett., 2017, 8(22), 5537–5542.

- TOOLS