Electrochemical Dopamine Sensors Based on Graphene
Article information
Abstract
The large surface area and the high electrical conductivity of graphene (GP) allow it to act as an “electron wire” between the redox center of biomolecules and an electrode surface. The faster electron transfer kinetics and excellent catalytic activity of GP facilitate the accurate and selective electrochemical detection of biomolecules. This mini-review provides an overview of the recent developments and progress of GP, functionalized or doped GP, and GP-composites based sensors for the selective and interference-free detection of dopamine (DA). The electrochemical principles and future perspective and challenges of DA sensors were also discussed based on GP.
1. Introduction
Graphene (GP) is the thinnest material in the universe, which is flexible, harder than diamond, superior conductivity to electricity at room temperature, high surface area (theoretically 2630 m2/g for single-layer graphene), excellent thermal conductivity, and high mechanical strength [1–4]. It has been considered as a “rising star” carbon material with elusive two-dimensional (2D) structure, where electrons obey a linear distribution relation and behave like a massless relativistic particle. These corroborate very peculiar electronic properties of GP, including quantum Hall effect [5] and transport via relativistic Dirac fermions [6]. It is also the basic structure of all graphitic materials with a one-atom-thick planar sheet of sp2 bonded carbon atoms in a honeycomb crystal lattice [3]. This outstanding physiochemical properties of GP lead to its applications in many technologies such as electronics [7], energy storage and conversion supercapacitors [8], batteries [9], fuel cells [10], solar cells [11], and bioscience/biotechnologies [12]. Particularly, GP and its composites have attracted a significant interest to the scientist and technologist as an electrode modifier for the development of different types of electrochemical biosensors to detect various biomolecules, including dopamine (DA), proteins, glucose, and cholesterol.
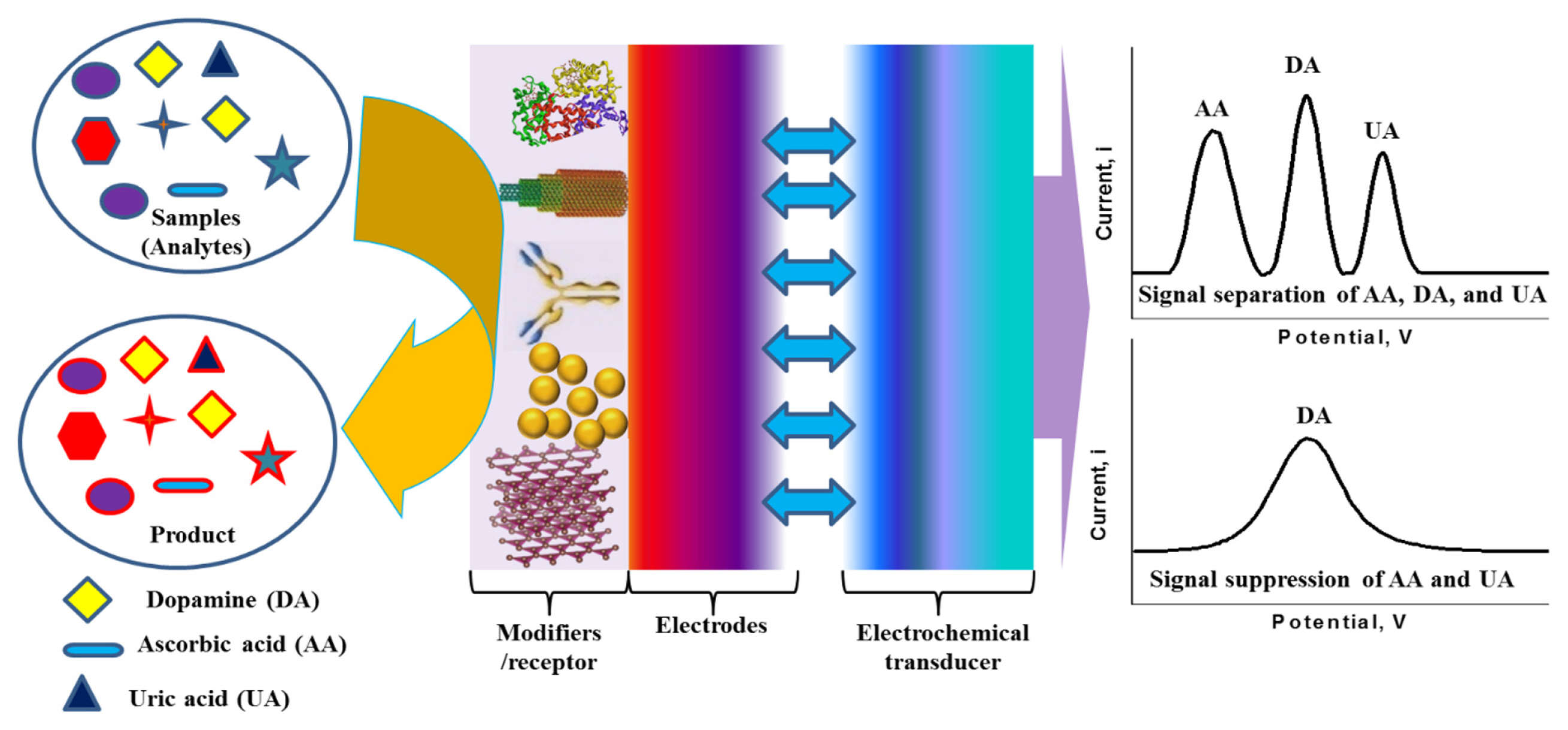
Dopamine (DA) is one of the major catecholamine neurotransmitter that plays the crucial role in the central nervous, renal, hormonal, and cardiovascular systems [13,14]. The unbalanced concentration of DA in the human body causes severe diseases such as Parkinson’s, and Schizophrenia [15]. Therefore, it is significantly important to measure the concentration of DA in human body fluids to get rid of those diseases as well as to regulate the body function properly. The electrochemical detection of DA is highly advantageous over other conventional techniques (e.g., chromatography, optical, and mass spectrometry) with respect to the sensitivity, selectivity, and simplicity [16–18]. The electrochemical oxidation potentials of DA and uric acid (UA) are very close. Most of the conventional electrodes (e.g., Au and Pt) cannot distinguish their oxidation potentials. While ascorbic acid (AA) also interfere significantly in the detection of DA due to its coexistence in biological fluids with a similar oxidation potential of DA. Various materials and methods have been suggested for the modification of the electrode surfaces to separate the signals of AA, DA, and UA or to suppress the signals of AA and UA from that of DA, including conducting polymers (CPs) [19–21], nanomaterials [12,22–24], covalent modification [25,26], self-assembled monolayers [27,28], and graphene [29]. Scheme 1 shows the general schematic diagram of an electrochemical sensor for the interference-free detection of DA. Particularly, GP is known to be a superior electrode modifier for the modification of electrodes surfaces to detect DA without the interferences of AA and UA [29]. Therefore, graphene oxide (GO), doped-GP, functionalized GP, and GP-composites were developed and used as electrode modifiers for the interferencefree electrochemical detection of DA with high sensitivity. This mini-review provides an overview of the electrochemical detection of DA based-on GP, doped-GP, functionalized GP, and GP-composites together with the electrochemical principles of GP-based DA sensors.
2. Electrochemical Principles of Dopamine Sensor
To date, numerous methodologies have been developed for the detection of DA, including electrochemical [30], colorimetry [31], conductometry [32], optical [33], and fluorescent spectroscopy [34]. Among them, electrochemical methods have attracted the highest attention due to its unbeaten sensitivity and selectivity. Additionally, electrochemical techniques exhibit a lower detection limit, faster response time, better long-term stability, low-cost, and miniaturization ability. Based on the principle of measurements, electrochemical DA sensors are classified principally as voltammetric and potentiometric sensors.
2.1 Voltammetric Dopamine Sensor
The current-potential (I-E) relationship of an electrochemical cell is the basis of voltammetric sensors. Amperometric sensors are also considered as the subclass of voltammetric sensors since it is also based on the I-E relationship of the electrochemical cell. In an amperometric sensor, a fixed potential is applied to an electrochemical cell for the redox reaction of DA. The corresponding current is measured to quantify DA, which is linearly proportional to the concentration of DA. A voltammetric sensor can operate in various modes such as cyclic voltammetric (CV), linear sweep voltammetric (LSV), square wave voltammetric (SWV), and differential pulse voltammetric (DPV). The respective I-E responses and the sensitivities of each mode are different [35–37]. All the voltammetric DA sensors examine the I-E characteristics for the redox reaction of DA based on its concentration variations. In general, the mass transfer rate of the analytes onto the electrode surface and the kinetics of the faradaic or charge transfer reaction at the electrode surface directly affect the I-E characteristics. This mass transfer can be accomplished by the ionic migration, diffusion, and bulk transfer [35–37]. The electrode reaction kinetics and the mass transfer processes contribute to the rate of the faradaic processes in an electrochemical cell. This provides the basis for the operation of a voltammetric sensor. The I-E curve usually shows a peak at a potential where the oxidation or reduction reaction occurs. The height of the peak current is used for the quantification of DA concentration. Among the voltammetric modes, amperometry and DPV are frequently used to quantify the concentration of DA due to their higher sensitivity.
2.2 Potentiometric Dopamine Sensor
When a redox reaction takes place at an electrode surface, a potential will develop at the electrode-electrolyte interface, which is used to quantify the concentration of analytes in a potentiometric sensor [38]. The potential difference at the electrode-electrolyte interface arising from unbalanced activities of analytes (i) in the electrolyte phase (s) and electrode phase (β), is related to the following Nernst equation:
Where, E0 is the standard electrode potential, R the gas constant, T the absolute temperature, F the Faraday constant, ai the activity of species i, and Zi the number of moles of electron involved.
Practically, potentiometric measurements need to be made under zero-current conditions since it operates at thermodynamic equilibrium conditions. Consequently, a high-input impedance electrometer is often used for the measurements. Also, the response time of a potentiometric sensor is quite long, which is due to the necessity of a long time to reach equilibrium conditions [39]. Therefore, a few reports are available for the potentiometric detection of DA [40].
3. Dopamine Sensor based on Graphene
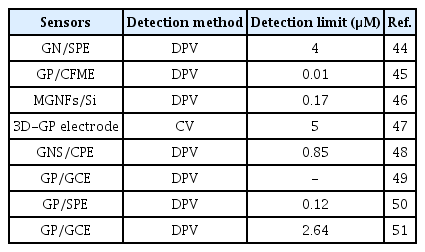
The electrochemical activity and the sensitivity of pure GP-based electrochemical sensors towards DA and many other analytes are highly dependent on the π-π stacking based interactions between the basal plane of GP and the analytes. Additionally, it also depends on the different electrochemistry-based redox interactions occurring at the defects edges of GP (e.g., kinks, steps, vacancies, and dangling bonds) [41–43]. The presence of a large number of defects at the edge plane of GP with sp2 domains facilitates the rapid and sensitive detection of DA better than carbon nanotubes (CNTs) [43]. For example, Valentini et al. developed a multianalyte sensor based on a functionalized graphene nanoribbon (GN)-modified screen-printed electrode (SPE). This GN/SPE sensor with defects at the edge plane of GN was used for the selective detection of potassium ferricyanide, catechol, hexaammineruthenium(III) chloride, sodium hexachloroiridate-(III) hydrate, DA, epinephrine, L-tyrosine, 3,4 dihydroxyphenylacetic acid (DOPAC), AA, UA, 4-acetamidophenol (acetaminophen), nicotinamide adenine dinucleotide (NADH), H2O2, caffeic acid, guanine, and serotonin (5-HT) hydrochloride [44]. Among them, DA showed very good sensitivity and detection limit with excellent reproducibility. Shang et al. developed a novel microwave plasma enhanced chemical vapor deposition method for the efficient synthesis of multilayer graphene nanoflake films (MGNFs) onto Si substrates. This MGNFs contained high graphitized knife-edge structure with a 2–3 nm thick sharp edge and showed a preferred vertical orientation with respect to the Si substrate [46]. The MGNFs/Si sensor exhibited faster electrontransfer kinetics for the Fe(CN)6 3−/4− redox system and excellent electrocatalytic activity for the simultaneous detection of AA, DA, and UA with improved sensitivity and a very low detection limit. Pure three-dimensional (3D)-GP based sensor was also developed by Xiao et al. [47], as shown in Fig. 1. This 3DGP structure was prepared on macroporous Ni foam scaffolds using a chemical vapor deposition (CVD) method. Ni scaffolds were removed by thermal annealing and etching process after the deposition of 3D-GP. This 3D-GP sensor showed the signal separation of AA, DA, and UA with the detection limit of 5 mM for DA detection. Various other GP modified sensors such as GP-modified carbon paste electrode (GP/CPE) and glassy carbon electrodes (GP/GCE) were also developed to improve the sensitivity and detection limit of DA [49–51]. Note that, some reported GP based sensors could easily suppress the electrochemical oxidation signals of AA and UA from that of DA, while others could easily separate the electrochemical oxidation signals of all. Based on the literature, it can be concluded that this signal suppression and separation issue is possibly dependent on the synthesis procedure and quality of GP (e.g., types of dopants or functional groups, and the percentage of dopants or functional groups), which require further studies. Table 1 shows the analytical performance of some reported GP-based sensors for the detection of DA.
4. Dopamine Sensor based on Functionalized/Doped Graphene
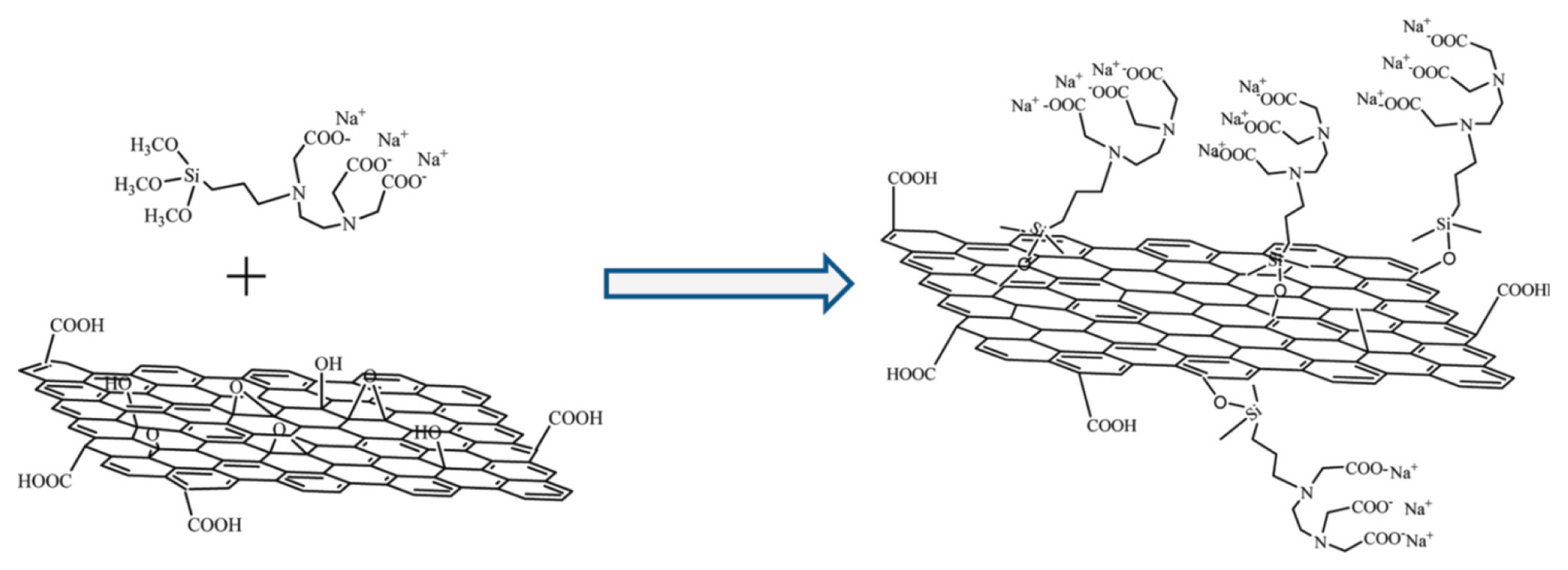
Chemical functionalization is the commonly employed strategies to improve the electrocatalytic activity of GP. The functionalization of GP with oxygen-containing functional groups (e.g., -COOH, - OH) induce to enhance the electron transfer kinetics as well as the catalytic activity for the oxidation of DA [44]. Synthesis of GO is the most commonly used oxygen functionalization strategy of GP, which was vastly used as an electrode modifier to detect DA. Gao et al. developed a GO modified GCE (GO/GCE), which could completely suppress the electrochemical oxidation of AA due to the electrostatic repulsion between the negatively charged oxygen functional groups (COO−) of GO with the negatively charged AA at pH 7.0. This GO/GCE sensor showed enhanced sensitivity for DA detection, as shown in Fig. 2 [52]. This is due to the improved electrostatic interaction with the positively charged DA at pH 7.0 with the negatively charged GO. Some other research groups also developed GO modified graphite electrode and GO micro-sheets for the detection of DA with enhanced sensitivity [53,54]. Wu et al. developed and porphyrin functionalized GP-modified GCE (porphyrin-GP/GCE) sensor for highly sensitive and selective detection of DA [55]. The aromatic π–π stacking and electrostatic attraction between positively-charged DA and negatively-charged porphyrin-modified GP could accelerate the electron transfer kinetics by weakening the oxidation signals of AA and UA. Similar electrochemical response of DA in the presence of AA was also observed by Hou et al. at a N-(trimethoxysilylpropyl) ethylenediamine triacetic acid (EDTA)-modified reduced graphene (RG) based sensor. This EDTA-RG was synthesized by the silanization of GP with EDTA-silane as shown in Fig. 3 [56]. Some other chemically functionalized GP such as sulfonated-GP and GP doped-film of layered double hydroxide (LDH) was also used for the detection DA [57,58]. However, the analytical performance of this sulfonated-GP and GP doped LDH based sensors were much lower compared to the porphyrin-GP and EDTA-RG based sensors. This can be attributed to the existence of a high percentage of negatively charged oxygen functional groups in these composites, which induces to enhance the interaction of positively charged DA. This concurrently enhances the sensitivity of DA detection.
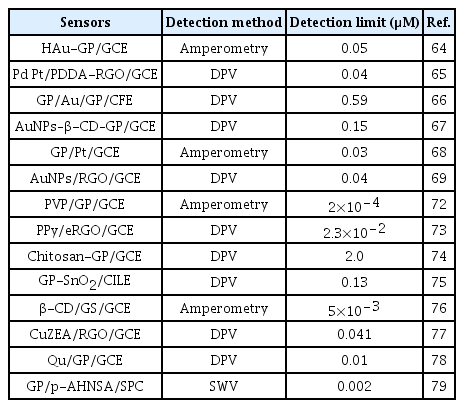
Application of doped GP is of high interest for the development of electrochemical devices since doping can tune its electrocatalytic properties significantly. Chemical doping with heteroatoms such as nitrogen, boron, sulfur, and phosphorous is an effective strategy to modulate electronic properties and surface chemistry of GP. For instance, nitrogen atoms can be doped easily into carbon structures via the formation of C-N bonds. This is due to the similarity of the atomic size and five valence-electrons of C and N. Sheng et al. prepared a nitrogen doped graphene (NGP)-modified GCE (NGP/GCE) electrode for the sensitive detection of DA [59]. The high electrocatalytic activity of this NGP/GCE sensor can be ascribed to the improved interaction of DA with NGP via hydrogen bond. This could activate the hydroxyl groups of DA and accelerate the charge transfer kinetics of DA oxidation at the NGP surface. This NGP/GCE sensor showed a very low detection limit (0.25 μM) with excellent sensitivities. Tan et al. developed a boron-doped GP electrode for the detection of DA and compared the results with a boron-doped diamond electrode (BDD) [60]. The boron-doped GP with sp2 bonded carbon atoms exhibited much lower oxidation potential than BDD electrode with sp3 bonded carbon atoms. These differences in the physiochemical properties of boron-doped GP and BDD could significantly control the sensitivity of DA detection, and it is much higher for the boron doped GP. Table 2 shows the analytical performance of some of the functionalized or doped GP modified sensors for the detection of DA.
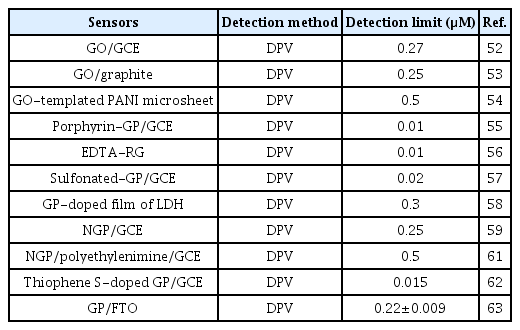
Analytical performances of some reported functionalized or doped graphene-based sensors for the detection of DA.
Considering the advantages of functionalization and doping of GP, our research group demonstrated the application of a COO− functional groups and N-doped GP-modified fluorine-doped tin oxide electrode (FTO) for the detection of DA [63]. This doped and functional GP showed very high electrocatalytic activity for the oxidation of DA at pH 7.0. This is due to the even better interaction of DA with this GP induced by the synergistic effect of N and COO− functional groups (Fig. 4). However, the detection limit (0.22±0.009 μM) for the detection of DA at this GP was little higher compared to the porphyrin-GP and EDTA-RG. This is due to the low-percentage of COO− functional groups (5.5%) and N-doping (1.5%) at the edges of this GP. Thus, controlling the percentage of oxygen functional groups and the heteroatom dopants at the edges of GP can be an effective strategy to enhance the performance of electrochemical devices as well as DA sensors.
5. Dopamine Sensor based on Graphene Nanocomposites
The tendency of monolayer GP to form graphite by agglomeration is the biggest challenge for the production of highly catalytic and conductive GP. The agglomeration of monolayer GP can be minimizing by preparing its composites with other nanomaterials, which could hinder the decrease of the analytical performance of sensors. Furthermore, the existence of nanomaterials into the GP composites could synergistically enhance the performance of sensors.
Recently, the application of metallic nanoparticles-GP hybrid is attracted much attention to the development of chemical sensors and biosensors. For example, Zhu et al. developed a hollow gold-GP (HAu-GP) nanocomposites-modified GCE electrode for the selective detection of DA [64]. The anchoring of HAuNPs onto the surface of GP sheets was achieved by mixing an aqueous solution of HAuNPs with a poly(N-vinylpyrrolidone)-functionalized GP dispersion at room temperature. The as-prepared HAu-GP/GCE sensor displayed a high electrocatalytic activity and extraordinary electron transport properties for the oxidation of DA with a nanomolar detection limit. Yan et al. developed a bimetallic (Pt-Pd)-GO composites for the signal separation of AA, DA, and UA, in which Pt and Pd ions were first attached to poly(diallyldimethylammonium chloride) (PDDA) functionalized GO sheets [65]. Then, the encased metal ions and GO were subjected to simultaneous reduction by ethylene glycol to prepare Pd3Pt1/PDDA-reduced graphene oxide (RGO) solution, which was drop cast onto GCE and applied for DA detection. This Pd3Pt1/PDDA-RGO/GCE sensor showed a nanomolar detection limit for the detection of DA. Some other research groups were developed layer-by-layer assembly of GP-gold nanoparticles (GNPs) (Fig. 5), β-cyclodextrin (β-CD)-GNPs-GP (Fig. 6), Pt-GP, and GNPs-RGO, GNPs and P-doped GP, and GP-conducting polymer (CP) composites for the detection of DA [66–70].
Recently, the preparation of GP and CP composites attracted significant interest to stabilize the GP at the electrode surface. It is well known that the conventional electrodes were usually modified with GP using Nafion or other nonconductive polymers (e.g., PVDF) as binders to improve the stability of the sensors. This is due to the low physical adhesion of pure GP with the conventional electrodes surfaces. However, these non-conducting binders can deteriorate easily the conductivity and catalytic activity of GP, which concurrently affect the performance of sensors. In this regard, the preparation of CPs/GP composites is an attractive approach to improve the stability of sensors, where CPs can serve as a binder and catalytic material. The synergistic effect of CPs and GP in the CPs/GP composite could increase the sensitivity of DA detection with high signal stability. For example, Liu et al. developed a poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) (PEDOT:PSS)/GP composites modified FTO electrode for the detection of DA with a low percentage of hydrophilic PSS in the composites [71]. This composite modified electrode showed high conductivity, catalytic activity, and stability for the oxidation of DA with the detection limit of 105 nM. In another report, Liu et al. developed a polyvinylpyrrolidone (PVP)/GP-modified GCE (PVP/GP/GCE) for the interference-free detection of DA [72]. Compared to a bare GCE, an increase of current signal was observed, demonstrating that PVP/GP/GCE exhibited favorable electron transfer kinetics and electrocatalytic activity towards the oxidation of DA. Furthermore, PVP/GP/GCE exhibited the ability to suppress the background current in the presence of an excess amount of AA. Si et al. fabricate polypyrrole (PPy) and GP composites thin film by the electrochemical polymerization of pyrrole with graphene oxide (GO) as a dopant, followed by electrochemical reduction of GO (eRGO) [73]. This PPy/eRGO composite-modified electrode exhibited high sensitivity and selectivity toward the detection of DA in the presence of high concentrations of AA and UA with a detection limit of 23 nM. A chitosan polymer functionalized GP was synthesized by blending and in situ chemical reduction of GP by Han et al. as shown in Fig. 7 [74]. The chitosan-GP-modified GCE showed high electrocatalytic activity towards oxidations of AA, DA, and UA with the detection limit of 2.0 mM for DA detection.
Metal oxides, zeolites, and dispersing agents based GP-composites also developed for the detection of DA. For example, Sun et al. prepared a GP-SnO2 composite modified carbon ionic liquid electrode (CILE) for the detection of DA [75]. The synergistic effects of GP and SnO2 in this nanocomposite induce to increase the sensitivity of the sensor with a detection limit of 0.13 mM. In another report, Tan et al. prepared a nanocomposite of β-cyclodextrin, a dispersing agent, and GP sheet (β-CD/GS), which exhibited high stability in aqueous solution [76]. The β-CD/GS modified GCE (β-CD/GS/GCE) electrode showed a wide linear range for the detection of DA with a good ability to suppress the background current in the presence of excess amount of AA. The electrochemical reaction of DA at the β-CD/GS/GCE sensor showed a mass diffusion-controlled process, which was different from the adsorption controlled process at the unmodified GS. Table 3 summarizes the analytical performance of some of the reported GP composites modified sensors for the detection of DA.
6. Conclusions: Future Prospective and Challenges
This mini-review presents the recent advances of the application of graphene (GP) for the electrochemical detection of dopamine (DA). All the GP-based DA sensors exhibited good sensitivity and selectivity towards the detection of DA in the presence of common interfering compounds of AA and UA with very low detection limits. GP is not only superior to conducting polymers, carbon nanotubes, and metal oxides for the electrochemical detection of DA, but also an efficient and inexpensive material for the large-scale manufacturing of sensors.
Based on the literature cited in this review article, it can be concluded that some GP could easily suppress the signals of AA and UA from that of DA, while others could separate all the three signals. The reason behind this behavior is still unclear. It can be suggested that suppression and separation behavior significantly depends on the extent of interaction of those biomolecules with GP, which can be controlled by controlling the quality and physiochemical properties of GP and it needs further studies.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01057380, NRF-2016R1A2B2012061).