Nitrogen and Fluorine Co-doped Activated Carbon for Supercapacitors
Article information
Abstract
Activated carbon has lower electrical conductivity and reliability than other carbonaceous materials because of the oxygen functional groups that form during the activation process. This problem can be overcome by doping the material with heteroatoms to reduce the number of oxygen functional groups. In the present study, N, F co-doped activated carbon (AC-NF) was successfully prepared by a microwave-assisted hydrothermal method, utilizing commercial activated carbon (AC-R) as the precursor and ammonium tetrafluoroborate as the single source for the co-doping of N and F. AC-NF showed improved electrical conductivity (3.8 S cm-1) with N and F contents of 0.6 and 0.1 at%, respectively. The introduction of N and F improved the performance of the pertinent supercapacitor: AC-NF exhibited an improved rate capability at current densities of 0.5-50 mA cm-2. The rate capability was higher compared to that of raw activated carbon because N and F co-doping increased the electrical conductivity of AC-NF. The developed method for the co-doping of N and F using a single source is cost-effective and yields AC-NF with excellent electrochemical properties; thus, it has promising applications in the commercialization of energy storage devices.
1. Introduction
Supercapacitors store and discharge electrical energy via the physical adsorption and desorption of ions at the electrode-electrolyte interface. This feature makes supercapacitors the most common energy storage media because of the semi-permanent charge/discharge cycle with high power density, non-requirement of special maintenance during their service life, and low “attendant-risk” of environmental contamination due to the use of carbon-based materials [1-6]. The characteristics of a supercapacitor are mainly determined by the electrode materials and the electrolyte [7], with the former being the subject of extensive investigations. Activated carbon exhibits a very large specific surface area, thereby leading to high specific capacitance, good chemical stability, and a low thermal expansion coefficient [3,7-11]. For these reasons, activated carbon is widely used as an active material for the electrode of a supercapacitor. However, activated carbon has lower electrical conductivity and reliability than those of other carbonaceous materials because of the oxygen functional groups formed during the activation process.
These drawbacks can be overcome by co-doping activated carbon with N and F, where the N and F atoms replace the O atoms in the oxygen functional groups. N doping can improve the electrical conductivity and wettability of carbon materials; F doping also improves the electrical conductivity by providing semi-ionic bonding characteristics in non-aqueous electrolytes [5,7]. Although N, F co-doping can improve the electrochemical performance of carbon electrodes [5,10-14], the doped activated carbons have generally been produced through hazardous high-temperature/pressure solvothermal processes that require separate N and F sources and organic solvents [5,13].
Herein, we present a low-temperature microwave-assisted hydrothermal method for the preparation of N, F co-doped activated carbon (AC-NF), by employing distilled water as the solvent and ammonium tetrafluoroborate (NH4BF4) as the single source for N, F co-doping. The structure, morphology, and surface functional groups of AC-NF are examined, and its electrochemical characteristics, such as volumetric capacitance and rate capability, are compared with those of conventional activated carbon. Through electrochemical tests, the potential application of AC-NF as an electrode material for supercapacitors is demonstrated.
2. Experimental
AC-NF was prepared by using commercial activated carbon (AC-R, YP50F) as the carbon source, NH4BF4 as the single source of both N and F, and distilled water as the solvent. Cetyltrimethylammonium bromide (CTAB) was additionally employed as the surfactant to promote N, F surface doping. Typically, AC-R (2 g) was dispersed in a solution of NH4BF4 (2 g) and CTAB (1 g) dissolved in distilled water (50 mL); the dispersed mixture was stirred for 6 h at room temperature. Subsequently, the suspension was transferred into a Teflon container, heated at 200℃ for 20 min in a hydrothermal microwave synthesizer, and cooled to room temperature. The solid product was separated by filtration, dried for 24 h at 120℃, heated at 900℃ for 1 h under nitrogen atmosphere, and finally cooled to room temperature to obtain AC-NF.
The N and F contents of AC-R and AC-NF, and their surface functional groups were identified by X-ray photoelectron spectroscopy (XPS; PHI 5000 VersaProbe, ULVAC-PHI Inc., Osaka, Japan). The specific surface areas were analyzed by the Brunauer-Emmett-Teller (BET) method based on nitrogen adsorption/desorption isotherms (Belsorp-mini II, Belsorp, Japan). Prior to obtaining the isotherms, the samples were outgassed at 200℃ for 12 h under vacuum. The total pore volume was determined from the amount of nitrogen adsorbed at p/p0 = 0.995. The electrical conductivity was measured at different pressures (30, 50, 70, 100 kgf cm-2) using a potentiostat (VSP, EC-Lab, France).
Rubber-type electrodes were prepared using AC-NF and a conventional (untreated) AC-R as the active materials, polytetrafluoroethylene (D-60, Daikin Industries, Japan) as the binder, and Super-P black (Timcal Graphite & Carbon Co., Belgium) as the conductive agent, in the weight ratio of 90:5:5. The abovementioned constituents were dispersed in ethanol by stirring until the solvent was almost completely volatilized. The resultant kneaded material was repeatedly rolled in a roll press machine heated at 60℃ to prepare a rubber-type electrode with a thickness of 200 μm. The obtained electrode was dried at 120℃ for 12 h and used as the working and counter electrodes. 2032-type coin cells were assembled using TP4035 (NKK, Japan) as the separator and 1 M tetraethylammonium tetrafluoroborate/propylene carbonate (1 M TEABF4/PC) as the electrolyte. Cyclic voltammetry (CV) experiments were performed in the potential window 0.0 to 2.7 V at various scan rates (1, 2, 5, 10, 20, 50 mV s-1), using a potentiostat (VSP, EC-Lap, France). Charge/discharge (CD) tests were performed in the voltage range 1.0-2.7 V (Δ = 1.7 V) at current densities of 0.5-50 mA cm–2 in the galvanostatic mode (HiEDLC-16CH, Human Instrument Co., Korea). The volumetric capacitance (Cvol) of the full cell was calculated as

where I, t, V, and W are the discharge current (A), time (s), potential window (V), and volume of the two electrodes (cc), respectively. Electrochemical impedance spectroscopy (EIS) measurements were performed between 100 kHz and 100 MHz using a potentiostat (VSP, EC-Lap, France).
3. Results and Discussion
The XPS spectra and elemental analysis results for AC-NF and AC-R are shown in Fig. 1a. Only C (94 at%) and O (6 at%) were present on the surface of AC-R, whereas N (0.6 at%) and F (0.1 at%) were additionally present on the surface of AC-NF. Moreover, AC-NF exhibited increased C content and decreased O content because of the substitution of the O atoms (that covered the surface dangling bonds of AC-R) by N and F atoms. Fig. 1b shows the deconvoluted N 1s spectrum of AC-NF, where each component has the same full width at half-maximum (FWHM) of 1.54 eV. Three types of N signals were observed in the spectrum: pyridinic N (397.9 eV), pyrrolic N (400.2 eV), and quaternary N (401.8 eV) [4,5,9,10]. The deconvoluted C 1s spectra of AC-NF and AC-R are shown in Figs. 1c and 1d, respectively, in which each component has the same FWHM of 0.94 eV. The C 1s spectrum of AC-R featured seven peaks attributed to carbide carbon (283.8 eV), C=C (284.4 eV), C–C (285.4 eV), C–O (286.3 eV), C=O (287.4 eV), and O–C=O (288.5 eV) bonds, with additional shake-up satellite peaks (289.9 eV) due to π–π* transitions in the aromatic rings [7]. For AC-NF, an additional peak corresponding to the C–F bond (289.3 eV) was observed, which was not detected in the case of AC-R. The relatively high C–F binding energy was due to the semi-ionic nature of this bond, arising from the large electronegativity difference between the constituents [5,7], which enhanced the conductivity of activated carbon by facilitating charge transfer between fluorine and carbon [7,15,16].
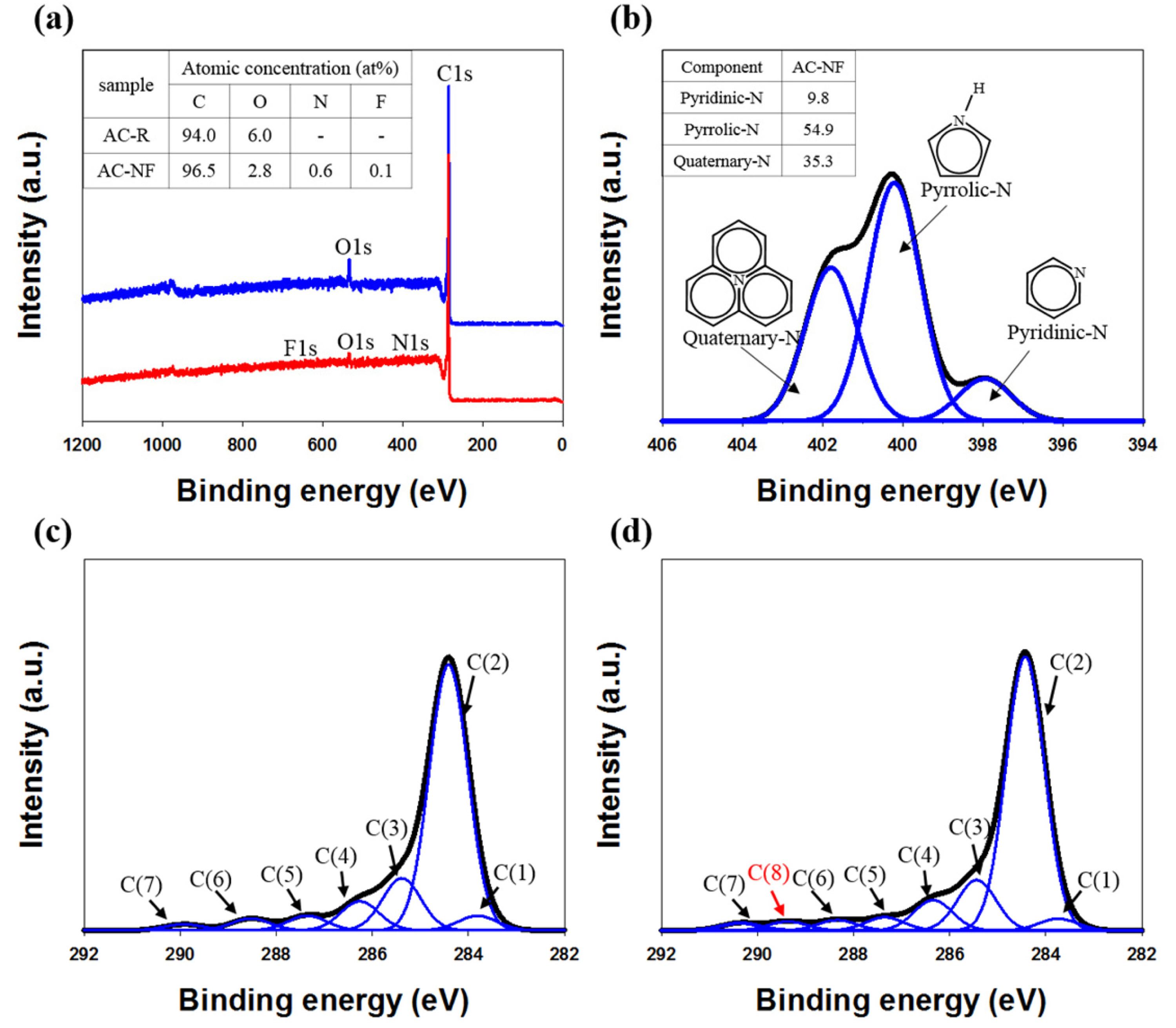
(a) XPS wide-scan spectra and elemental analysis results for raw (AC-R) and N, F co-doped (AC-NF) activated carbon, (b) N 1s narrow-scan spectrum of AC-NF, (c) C 1s narrow-scan spectrum of AC-R, and (d) C 1s narrow-scan spectrum of AC-NF.
Fig. 2a shows the nitrogen adsorption/desorption isotherms of AC-R and AC-NF, which show characteristic type I (IUPAC) features with well-defined plateaus, suggesting the microporous characteristics of the samples [17]. From BET analysis, the specific surface areas of AC-R and AC-NF were determined to be 1668 and 1480 m2 g-1, respectively. Fig. 2a shows that N, F co-doping reduced the specific surface area of AC-NF by 188 m2 g-1; the pore volume of AC-NF was 0.68 cm3 g-1, which was 0.07 cm3 g-1 smaller than that of AC-R. This could be attributed to micropore clogging or structural collapse after the thermal treatment [14]. As shown in Fig. 2b, the electrical conductivity of AC-NF (3.8 S cm-1 at 100 kgf cm-2) was higher than that of AC-R (2.0 S cm-1 at 100 kgf cm-2). This supported a previous claim that the electrical conductivity of activated carbon is improved by N, F co-doping [5,7,13].
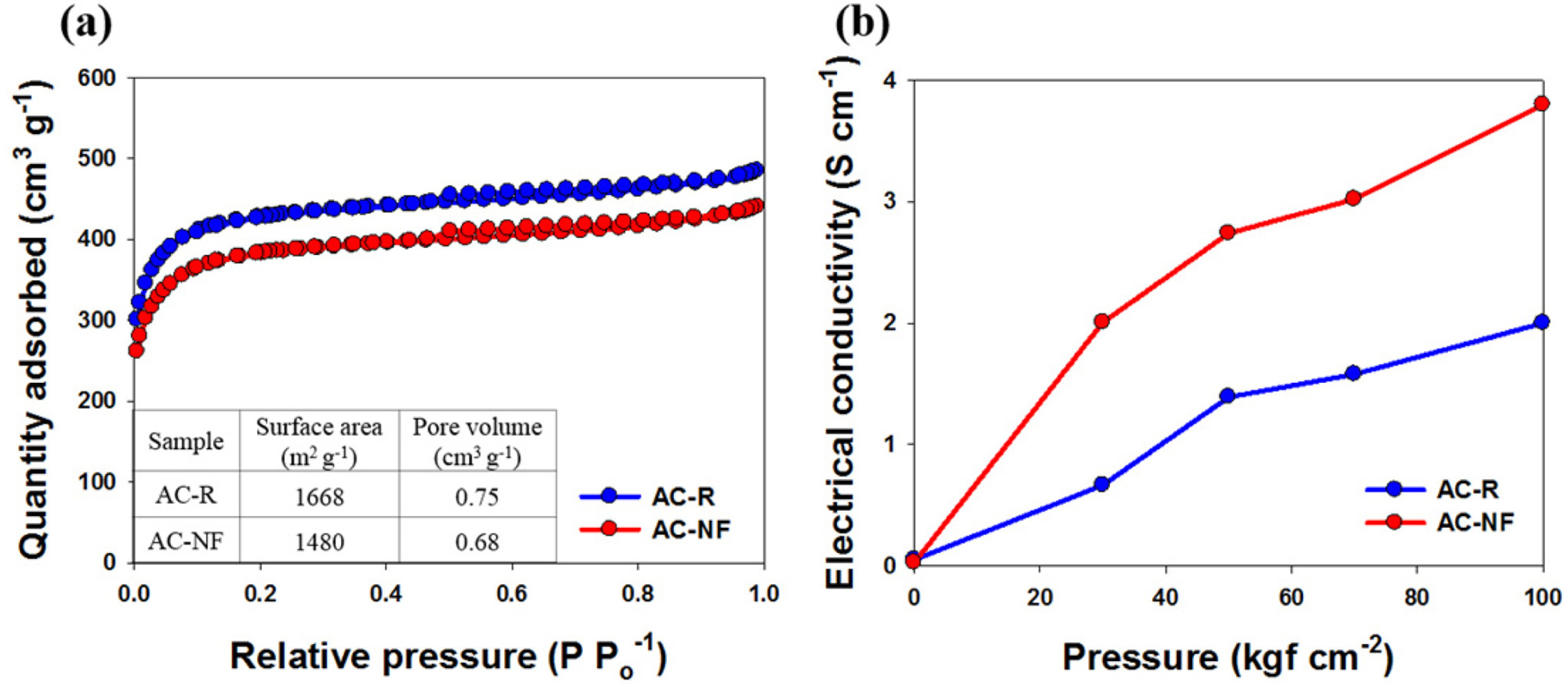
(a) Nitrogen adsorption/desorption isotherms of AC-R and AC-NF. (b) Electrical conductivities of AC-R and AC-NF as functions of pressure.
Fig. 3 shows the results of electrochemical tests performed using the 2032-type coin cells with 1 M TEABF4/PC as the electrolyte. First, CV profiles were acquired at different scan rates (1-50 mV s-1) in the voltage range 0.0-2.7 V to evaluate the capacitive performance of AC-R (Fig. 3a) and AC-NF (3b). For AC-NF, the volumetric capacitances were 13.6 F cc-1 at 1 mV s-1, 13.6 F cc-1 at 2 mV s-1, 13.4 F cc-1 at 5 mV s-1, 13.2 F cc-1 at 10 mV s-1, 12.7 F cc-1 at 20 mV s-1 and 11.6 F cc-1 at 50 mV s-1. In addition, the capacitance retention at a scan rate of 50 mV s-1 was 85% of the value at 1 mV s-1, which was 7% higher than that of AC-R. At a high scan rate of 50 mV s-1, the cyclic voltammogram of AC-NF was closer to rectangular than that of AC-R. This indicated the high electrical conductivity and wettability of AC-NF due to N, F co-doping. The results of the charge/discharge tests at various current densities (0.5-50 mA cm-2) are shown in Fig. 3c. Because of the increased mobility of charge carriers on the carbon surface, resulting from N, F co-doping, the capacitance difference between AC-NF and AC-R is not large despite the decreased specific surface area [13]. The volumetric capacitances of AC-NF were 14.6 and 10.8 F cc-1 at current densities of 0.5 and 50 mA cm-2, respectively, which corresponded to 12% higher capacitance retention than that of AC-R (Fig. 3c). The EIS data (Fig. 3d) showed that the improvement in the electrical conductivity due to N, F co-doping contributes to a reduction in the charge transfer resistance of AC-NF.
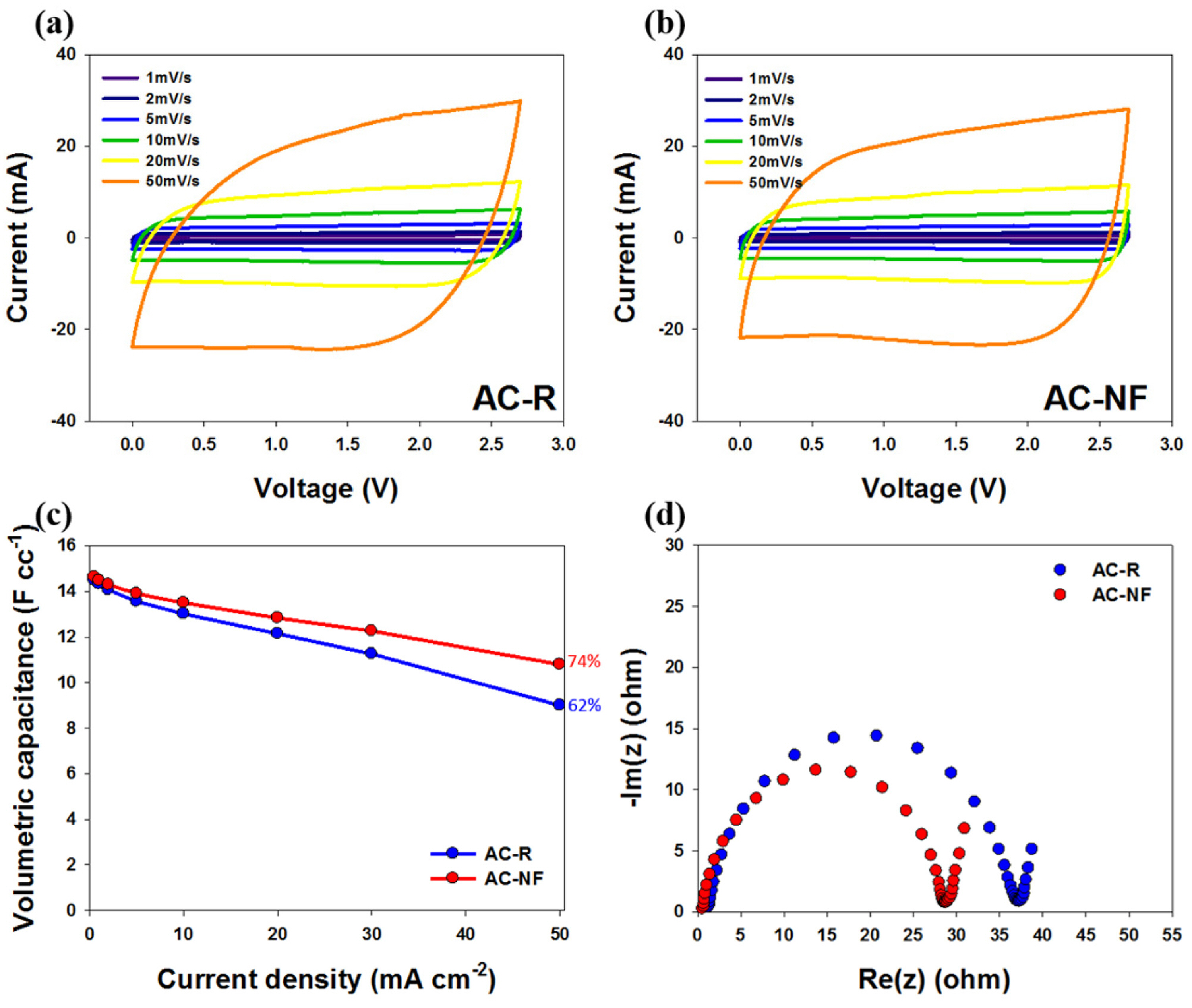
Cyclic voltammograms of (a) AC-R and (b) AC-NF at various scan rates, in the voltage range 0.0-2.7 V, (c) Rate capabilities of AC-R and AC-NF at various current densities, in the voltage range 1.0-2.7 V. (d) Nyquist plots for AC-R and AC-NF recorded at frequencies between 100 kHz and 100 MHz.
Thus, the enhanced supercapacitor performance of AC-NF could be explained as follows: the incorporation of N and F improved the electrical conductivity by ensuring rapid charge transfer, increased electrolyte ion accessibility, reduced internal resistance, and allowed sufficient utilization of the surface area. This led to high capacitance retention of AC-NF even at high current densities or scanning speeds.
4. Conclusions
N, F co-doped activated carbon has been successfully prepared by a microwave-assisted hydrothermal method using commercial activated carbon as the precursor and NH4BF4 as the single source for N, F co-doping. This material exhibits high electrical conductivity and improved electrochemical performance. This cost-effective technique for simultaneous N, F codoping holds great promise for the commercialization of advanced electrode materials for supercapacitors.
Acknowledgements
This work was supported by the industrial promotion program of economic cooperation area of MOTIE/KIAT (R0004005, development of EDLC for high-temperature (140℃) application. And this work was supported by a grant from the Fundamental R&D program and funded by the Korea Institute of Ceramic Engineering and Technology (KICET) and Ministry of Trade, Industry and Energy (MOTIE), Republic of Korea (No.KPP15008).