1. Introduction
We previously reported the simple preparation of polysiloxane viologen polymer/diaphorase/hydrophilic polyurethane (PSV/DI/HPU) modified electrode for sensing NAD+ and NADH.1) The sensitivity for NAD+ detection was 0.2 nA┬Ę╬╝MŌłÆ1 and the detection limit was 28 ╬╝M with a response time of 50 s (t90’╝ģ). In this case the overcoating of HPU trapped the enzyme and mediator, but it might hinder the transportation of NAD+ and NADH to decrease the sensing capability. Therefore, in this paper, we tried to immobilize the enzyme, diaphorase (DI) covalently with mediators without overcoating. The derivatized viologen, propylamine viologen (PAV) was synthesized and poly(ethylene glycol)(400) diglycidyl ether (PEGDGE), which is a crosslinker for amine, was used to crosslink PAV and DI. Amine groups in propylamine viologen and diaphorase enzyme reacted by nucleophilic addition to the epoxide ring in PEGDGE and the crosslinked PAV/PEGDGE/DI structure forms and precipitates on the electrode surface.2) The sensitivity of this system for NAD+/NADH detection increased dramatically as we expected.
2. Experimental
2.1 Reagents
Diaphorase (NADH: dye oxidoreductase) was purchased from Roche in Germany and ╬▓-NAD+ sodium salt from Yeast and NADH were from Sigma. Poly(ethylene glycol (400) diglycidyl ether) (PEGDGE) was purchased from Polyscience, Inc. Propylamine viologen (PAV) monomer was synthesized as described in the next section. For electrochemical experiments, phosphate buffer solution (0.1 M potassium phosphate monobasic pH 7.0, PB) was used. All aqueous solutions were prepared using distilled/deionized water (18 M╬®┬Ęcm). When measuring electrocatalytic activity, the concentrations of NADH and NAD+ in solution were changed by adding incremental amount of concentrated NADH and NAD+ solution in 0.1 M PB at pH 7.
2.2 Synthesis of propylamine viologen (PAV) monomer
3-Bromopropylamine hydrobromide (5.12 mmol) was dissolved in acetonitrile (10 mL) and 1-methyl-4,4-bipyridyl (5.12 mmol in 10 mL methanol) was added to the solution. The mixture was refluxed at 85Ōäā for over 6 hours. After the reaction, the mixture led to precipitation of aminopropyl viologen as yellow crystals. The compound was purified by the reprecipitation in the mixture of water/methanol = 1/1 and the yield was 75’╝ģ.
2.3 Preparation of PAV/PEGDGE/DI modified electrode
Printed carbon electrode (5 mm diameter, Zensors, Co.) was treated with air plasma for 10 min. Diaphorase enzyme solution of 20 mg/mL was prepared in 0.1M PB, pH 7. Propylamine viologen monomer of 10 mg/mL was made in 0.01 M tris buffer, pH 8.5, and PEGDGE was diluted to 50 mg/mL in 0.01 M tris buffer, pH 8.5. The equal volume of each solution were mixed completely and 2 ╬╝L of the mixture was pipetted to the electrode surface. The electrode was dried in room temperature for a day and stored at ŌłÆ4Ōäā until use. The modified electrode was washed with the buffer at least twice before measuring the electrochemical measurements.
2.4 Electrochemical measurements
CHI 1000 potentiostat (CH instruments, USA) was used for the electrochemical measurements. The PAV/ PEGDGE/DI modified working electrode, coiled platinum wire counter electrode, and Ag/AgCl (saturated KCl) reference electrode were used in a single-compartment cell. In amperometric detection, the aliquots of a stock solution of NAD+ or NADH was added to the solution under constant stirring after the background current reached to a steady state value.
3. Results and Discussion
3.1 Cyclic voltammograms of the PAV/PEGDGE/ DI modified electrode
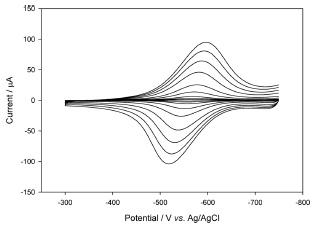
Cyclic voltammograms of the PAV/PEGDGE/DI modified electrode at different scan rates in the potential range of ŌłÆ0.3 V to ŌłÆ0.75 V in 0.1 M PB are shown in Fig. 1. Well-defined reduction and oxidation peaks of surface-confined reversible reaction were observed. The cathodic and the anodic peak potentials shifted a little as increasing the scan rate and the peak separation is only 60 mV at 250 mV/s. Both imply the fast electron transfer kinetics of the modified electrode. The peak current increases linearly to the scan rate at low scan rate (2-50 mV/s) but to the square root of scan rate at high scan rate (50-250 mV/s). It can be explained by the surface confined reaction is limited by the diffusion of balancing ions upon the electron transfer at high scan rate. The redox active viologen moiety is confined in the polymer matrix and collisional electron transfer occurs in the hydrogel and the ion diffusion for the charge balance is often the limiting step.2)
3.2 Electrocatalytic reduction of NAD+ on PAV/ PEGDGE/DI modified electrode
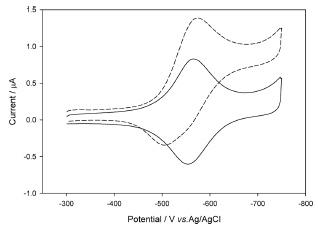
Figure 2 shows the cyclic voltammograms of the modified electrode in a phosphate buffer solution in the absence and the presence of 0.2 mM NAD+. The increase of the cathodic current near ŌłÆ0.6 V upon the addition of NAD+ clearly shows NAD+ is catalytically reduced on PAV/PEGDGE/DI modified electrode. Note that only 0.2 mM addition of NAD+ shows the significant increase in the catalytic current which is comparable to the addition of 3 mM in the previous result which used the soluble mediator with HPU coating.1) It clearly shows that the PAV/PEGDGE/DI polymer composite without coating enhances the catalytic effect mainly by the free movement of the substrate and product, NAD+ and NADH.
3.3 Electrocatalytic oxidation of NADH on PAV/ PEGDGE/DI modified electrode
Diaphorase is known to be a reversible catalyst for NAD+/NADH conversion3) and the electrocatalytic oxidation capability of the PAV/PEGDGE/DI layer was tested. The voltammetric response of the electrode clearly shows that the catalytic oxidation occurs (Fig. 3). Therefore, the PAV/PEGDGE/DI electrode can be utilized for both NAD+ and NADH sensor.
Fig.┬Ā3.
Cyclic voltammograms of the bioelectrocatalytic NADH oxidation at the PAV/PEGDGE/DI modified electrode in 0.1 M, PB (pH 7) at 2 mV/s in the absence (solid line) and in the presence (dotted line) of 0.1 mM NADH.

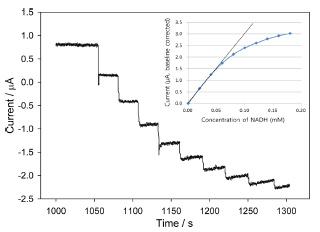
The amperometric response of the modified electrode for NADH oxidation was measured at ŌłÆ0.45 V vs. Ag/AgCl (Fig. 4). The oxidation current increases upon 0.02 mM incremental addition of NADH with a response time of less than 2 s (t90’╝ģ). Note that the response time of the previous report having HPU overcoating was 50 s. Upon analyzing the data, the sensitivity of the PAV/PEGDGE/DI electrode is determined to 0.02 nA┬Ę╬╝M-1 and the detection limit is about 3 ╬╝M (S/N = 3). More than 20 times increase in the response time (2 vs. 50 secs) and ~10 times increase in the sen-sitivity (0.02 vs 0.2 nA┬Ę╬╝M-1) also confirm that the catalytic behavior of DI-incorporated layer is significantly increased upon the removal of the HPU overcoating layer.
3.4 pH effect in the electrocatalytic oxidation of NADH and reduction of NAD+
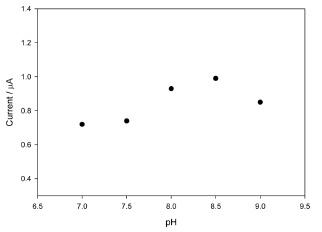
The pH dependence of catalytic oxidation the PAV/ PEGDGE/DI modified electrode was examined in the range of 7 to 9 (Fig. 5). The pH effect is not significant in the pH range, but the optimal pH for the detection is around 8 to 8.5. Since the pH optimum for diaphorase is known to be 8.5,4) the optimum pH for the detection is mainly governed by the activity of the enzyme.
4. Conclusions
We demonstrated that the PAV/PEGDGE/DI modified electrode greatly enhanced the catalytic effect for NADH/NAD+ conversion compared to the previously reported one which contains the HPU over-coating. The stable immobilization of diaphorase with mediator by covalent linkage is a key improvement in the sensitivity which made the free movement of NAD+ and NADH to the catalytic layer without the trapping layer.