Performance Analysis with Various Amounts of Electrolyte in a Molten Carbonate Fuel Cell
Article information
Abstract
The effect of initial electrolyte loading (IEL) on cell performance in a coin-type molten carbonate fuel cell (MCFC) was investigated in this work. Since the material of MCFC depends on the manufacturer, optimisation requires experimental investigation. In total, four IEL values, 1.5, 2.0, 3.0, and 4.0 g, were used, corresponding to a pore filling ratio (PFR) of 38, 51, 77, and 102%, respectively. The cell performance with respect to the PFR was analysed via steady-state polarisation, step-chronopotentiomtery, and impedance methods. The electrochemical analyses revealed that internal resistance and overpotential of the cell decreased with increasing PFR, and a large overpotential was observed when the PFR was 102%, probably due to the flooding phenomenon. After operation, cross-section of the cell was analysed via surface analysis of SEM and EDS methods, and the remaining electrolyte was estimated by dissolution of the cell in 10 wt% acetic acid. A linear relationship between IEL and the weight reduction ratio by dissolution was obtained. Thus, the remaining amount of electrolyte could be measured after operation. The results of SEM and EDS showed that a PFR of 38 and 102% showed a lack and flooding of electrolytes at the cell, respectively, which led to a large overpotential. This work reports that MCFC performance is allowed only in the narrow range of PFR.
1. Introduction
A Molten Carbonate Fuel Cell (MCFC) is expected to be a high-efficiency power generation system which converts the chemical energy of a fuel directly into electrical energy. The high efficiency of MCFC basically originates from the high operating temperature of 580-700 ℃ [1-4]. Thus, MCFCs have many advantages, including not requiring a precious metal as an electro-catalyst, as well as fuel flexibility, and low material cost [5]. These characteristics make MCFCs a key emerging technology for meeting future energy demands [6]. For more applications, it is essential to improve cell lifetime and cell performance [7].
MCFCs use molten carbonate as an electrolyte. When the temperature increases over 500 ℃, the carbonates melt and become ionic conductor (CO32−) [1-3]. The molten salts prevent gas leakage at the electrodes by sealing the matrix [8-9]. However, the high operating temperature of MCFCs can cause severe corrosion of components, which leads to performance degradation. In a previous work [7], it was reported that the degradation caused by electrolyte depletion resulted in an increase of internal resistance and electrode polarisation in a bench-scale MCFC. It is a relatively slow process. Therefore, a key point affecting the durability of a MCFC is the amount of electrolyte in the cell. It is determined by the degree of filling in the electrode which affects the polarisation of electrodes. The optimal filling of porous electrodes is decisive for MCFC performance [10]. The MCFC cathode, consisting of in situ oxidised NiO, is wetted completely by molten carbonate due to the very low contact angle [11]. Thus the cathode is entirely covered with a relatively thick film. This causes diffusion resistance of active species at the electrolyte [9]. When the pores become filled with electrolyte, diffusion resistance increases steeply due to the flooding phenomenon. Selman et al. reported that the cathode is especially sensitive to the degree of filling, where the optimum operating condition is 15-30% [11].
On the other hand, the anode has a small fraction of micropores which are partly filled with electrolyte, and work as a carbonate reservoir [12]. The anode is considered to be a dry condition because the anode, with a higher contact angle than the cathode, is poorly wetted by the carbonate electrolyte [8-9]. In addition, the anode has less dependence on the degree of filling because its electrode kinetics and mass-transport are relatively rapid. Therefore, the anode is much less sensitive to electrolyte filling than the cathode [8,12].
In a previous work, a bench-scale cell aiming for long-term operation at 600 ℃ was investigated [13]. The cell was operated for 66,000 h while receiving 7 electrolyte additions (2 g per addition). During operation, it was found that the electrolyte amounts controlled the internal resistance and cell performance. Therefore, it is considered that electrolyte loading is one of the vital factors to determine the cell performance.
In this work, performance with various amounts of initial electrolyte loading (IEL) was electrochemically investigated in a coin-type MCFC. Post-analysis of cell components after operation was also chemically investigated.
2. Experimental Section
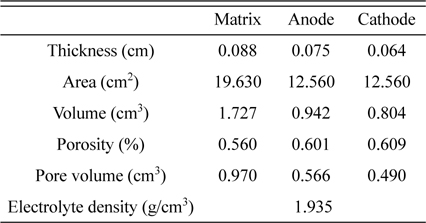
In this work, a coin-type molten carbonate fuel cell was used to minimise material consumption. The diameter of the electrodes was ca. 3 cm. The anode was a porous Ni-Al metal sheet, and the cathode was a porous in situ oxidised NiO. The matrix was made of LiAlO2, and the electrolyte was a eutectic mixture of 62 mol% Li2CO3 and 38 mol% K2CO3 [14]. The electrodes and matrices were supplied by the Korean Institute of Science and Technology (KIST).
The anode gas was a mixture of 71 mol% H2, 15 mol% CO2, and 14 mol% H2O. Flow rate of each gas was 125 mL/min H2 and 25 mL/min CO2 and these gas flows through a humidifier of 53 ℃, corresponding to 14 mol% H2O. A gas mixture of 70 mol% air and 30 mol% CO2 served as the cathode gas. All of the cells were operated at 650 ℃ under atmospheric conditions.
The cell performance was electrochemically analysed via steady-state polarisation (SSP), step-chronopotentiometry (SC), and electrochemical impedance spectroscopy (EIS). The SSP measures the correlation between current and voltage from 0 to 150 mA/cm2 of current density. The SC involved increasing the current from 0 to 150 mA/cm2 by 50 mA/cm2 step for 60 s and measuring the voltage relaxations [14], while the EIS measures the impedance by changing the AC signalling of 5 mV RMS from the high frequency (10 kHz) to low frequency (0.01 Hz) at an open-circuit state. All electrochemical ways were carried out after 12 h of cell operation.
This work focused on the effect of the electrolyte amounts on the cell performance and investigated the correlation between electrolyte amounts and cell performance via post-analysis after the cell operation. Four different amounts of electrolyte, 1.5, 2.0, 3.0, and 4.0 g, were used. The electrolyte was supplied to the anode in a form of powder through the anode chamber at around 297 ℃. At temperatures over 500 ℃ the powder was melt and distributed to all electrodes. The cell was operated for 12 h, and after the cell operation; one part of the cell was used for surface analysis. Scanning electron microscopy (SEM, JSM-6390) and energy dispersive spectroscopy (EDS, ISIS) were employed for electrolyte distribution. The other part of the cell was used to measure the remaining amount of electrolyte. The cell components were dipped into 200 mL of 10 wt% acetic acid solution for 24 h, and the weight reduction ratio before and after operation was measured. Since acetic acid can only dissolve the carbonate electrolyte, the reduction ratio is solely attributed to the reduction of carbonate amount. Thus, the different reduction ratios according to the initial electrolyte loading indicate the amount of carbonate remaining inside the cell.
3. Results and Discussion
The carbonate amount is expressed by pore filling ratio (PFR) which is the filling ratio of initial electrolyte volume to the total pore volume (anode, cathode, and matrix). In general, a proper electrolyte amount is necessary to provide an active surface area so that electrode reaction can take place on the surface. However, the cell performance can be reduced due to the flooding phenomenon which closes the pores by the electrolyte when the levels are over a certain threshold. The PFR is defined as the following:
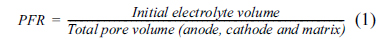
Thus, PFR can be obtained by using data of Table 1 and initial electrolyte volume. The density of Li/K carbonate electrolyte is 1.935 g/cm3. The electrolyte amounts of 1.5, 2.0, 3.0, and 4.0 g correspond to 38, 51, 77, and 102 % PFR, respectively.
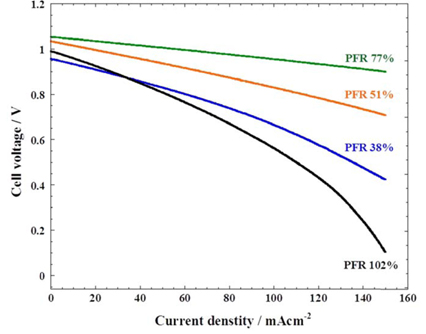
Fig. 1 shows the SSP behaviours with various amounts of electrolyte at 650 ℃. The current density was applied from 0 to 150 mA/cm2. The theoretical open-circuit voltage (EOCV) of hydrogen fuel can be expressed by the following Nernst equation (Eqn 2):
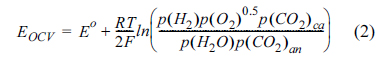
where Eo is the standard potential; and the subscripts an and ca denote the anode and cathode, respectively. Eqn (2) represents the theoretical maximum voltage of the cell, and the real voltage can be expressed by the difference between EOCV and total voltage loss. The output voltage has the following relationship:

where ηtot = ηIR + ηan + ηca, ηIR is the Ohmic loss, and ηan and ηca are the overpotential due to the electrochemical reactions at the anode and cathode, respectively [15]. By increasing current density, a decrease of the cell voltage is observed due to the increasing overpotential. Thus, total voltage loss can be estimated by using the SSP method. The standard voltage, Eo, for the oxidation of hydrogen is 1.03 V at 650 ℃ [16]. The EOCV is affected only by the partial pressure of reactant gas according to Eqn (2). Thus, the value of EOCV should be obtained by the same gas condition. However, the measured EOCV of the cells show a slight deviation, due to experimental error. The electrode reaction takes place at the active surface area of the three-phase boundary of gas-liquid-solid, and the area is widened by increasing electrolyte amounts. At a PFR of 77%, total voltage loss was 151 mV at a current density of 150 mA/cm2. A linear decrease in the voltage at lower current density was observed from the behaviour of voltage, indicating that the PFR 77% has negligible activation polarisation. When the electrolyte amount increases from 38% to 77% PFR, total voltage loss decreases from 532 mV to 151 mV. This indicates that increasing the electrolyte amount widens the active surface area, and reduces charge and masstransfer resistance. On the other hand, a severe voltage loss was observed at the PFR 38% and 102%. The small amount of electrolyte at PFR 38% leads to a reduction of the active surface area, while too high a level of electrolyte at PFR 102% results in flooding of the electrodes. Those resulted in a significant increase of mass-transfer resistance.
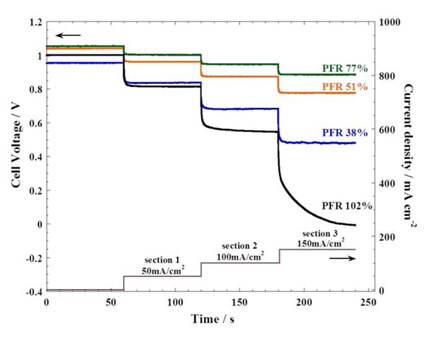
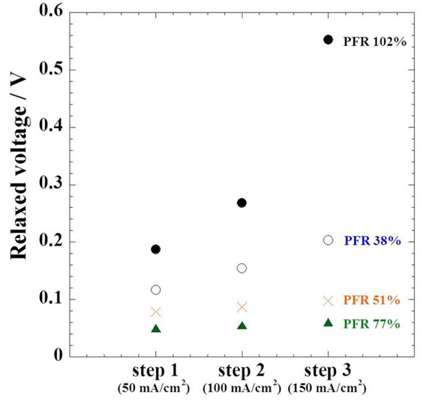
Fig. 2 shows SC behaviours with various amounts of electrolyte at 650 ℃. All cells show step-like voltage relaxation by the current steps. The SC method reveals reaction characteristics by the voltage relaxation [17]. The voltage relaxation was reduced by the increase in electrolyte amount up to PFR 77 %, and slow voltage relaxation was observed above and below the PFR value. At PFR 102 %, voltage relaxation slowed dramatically. In the fuel cells, the voltage relaxation comprises Ohmic loss ascribed to the electrical contact resistance between the cell components and overpotential due to the charge and masstransfer resistance in the electrochemical reactions. When current density increases, voltage relaxation is affected by Ohmic loss and mass-transfer resistance. The mass-transfer resistance includes anodic and cathodic overpotentials. If the cell is affected only by Ohmic loss, voltage relaxation of each step should theoretically be the same. In this work, however, voltage relaxation of each step was varied. This means that other resistance factors are involved in voltage relaxation. Fig. 3 shows the measured value of each voltage relaxation from Fig. 2. At the 77 % PFR, voltage relaxation was ca. 55 mV and showed consistent values at whole current steps. This means that the PFR of 77 % has the smallest overpotential among PFR values. On the other hand, at the PFR 38 and 102 % the voltage relaxation of each step increased by rising the current density. In the case of the 38 % PFR, the voltage relaxation value increased slightly from 116 mV to 204 mV. However at the PFR of 102%, the voltage relaxations in each current step were 187, 268, and 552 mV. Thus, the SC results represent mass-transfer effect on the voltage relaxation in addition to the Ohmic loss.
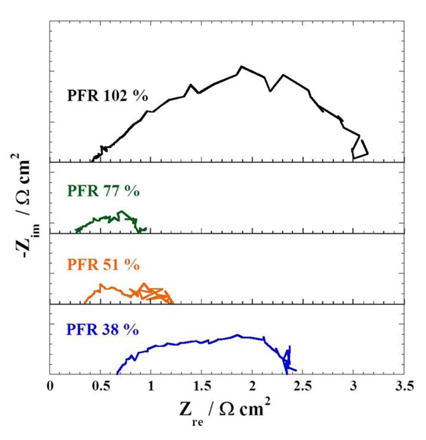
Fig. 4 shows EIS behaviours of the cells. In a previous work [18], two half circles at high and low frequencies were observed; the high frequency one reflected cathodic overpotential due to the masstransfer resistance through the liquid electrolyte at the cathode, and the low frequency one represented anodic overpotential. It is reported that the real value of the highest frequency represented the internal resistance. In Fig. 4, internal resistance decreased from 0.67 to 0.26 Ω·cm2 by enlarging the electrolyte amount to PFR 77 %, while a PFR of 38 % and 102 % showed larger internal resistance and half circles than PFR 77 %. It is plausible that electrolyte amounts that are too large or too small reduced the active surface area and resulted in large mass-transfer resistance and voltage relaxations. In particular, the large half circle of 102 % PFR is attributed to flooding at the electrodes. Moreover, a large resistance gap was observed between PFR 38 % and 51 %, although the difference in electrolyte amount was only 0.5 g. This suggests that a permissible range of electrolyte is limited and the performance is very sensitive to the degree of filling in an MCFC.
Fig. 5 shows EDS images with various amounts of electrolyte. (a), (b), (c), and (d) are PFR 38 %, 51 %, 77 % and 102 %, respectively. The white dots in the EDS images represent distribution of electrolyte. In this work, electrolyte was supplied to the anode, thus the electrolyte moved from the anode to the cathode. For this reason, the PFR 38 % shows that the electrolyte was poorly distributed to the cathode, as shown in Fig. 5a. However, by enlarging the amount of electrolyte, it was distributed at the cathode, as seen in Figs. 5c and 5d. A previous study by Fontes et al. [19] reported that the performance of the MCFC is mostly determined by the kinetics of the cathode. On the basis of the results of EDS and impedance, it is found that the PFR 38 % has a large half circle. This means that the cathode was poorly wetted by the electrolyte, which led to a decrease of active surface area of the gas-liquid-solid three-phase boundary. Thus, it can be assumed that mass-transfer resistance was significant at the PFR of 38 %. However, the PFR of 102 % shows full electrolyte distribution at the cathode, indicating that excess amounts of electrolyte induced severe mass-transfer resistance by the flooding phenomenon.

EDS mapping image (× 3000) of each cell, (a) PFR 38%, (b) PFR 51%, (c) PFR 77%, (d) PFR 102%, the white dots on EDS images show distribution of the electrolyte element K.
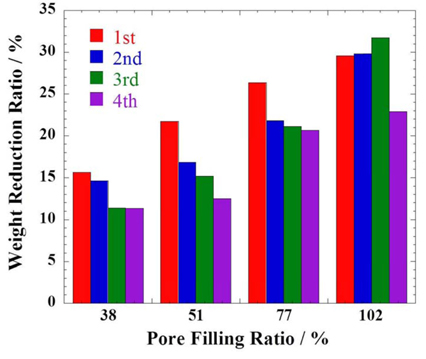
Fig. 6 shows a correlation between the weight reduction ratio of components after operation into acetic acid and pore filling ratio. Since the carbonate electrolyte is well dissolved in acetic acid, the weight reduction at the acetic acid solution would have a relationship with the remaining electrolyte. In this work, four initial electrolyte loadings, 1.5, 2.0, 3.0, and 4.0 g, and 16 samples were tested in total. Although the weight reduction ratio has some deviation, it increases by enlarging electrolyte amounts. Indeed, the cell components are comprised of electrodes, matrix and electrolytes, and the acetic acid dissolves the electrolytes only. Thus higher electrolyte loading results in larger reduction ratio. The average weight reduction ratio was 13.23, 16.58, 22.49, and 28.49 % at 1.5, 2.0, 3.0 and 4.0 g, respectively. Thus, it is possible to estimate the electrolyte remaining in the cell after operation.
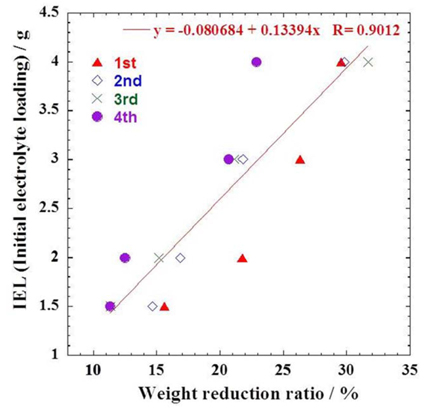
Fig. 7 shows a correlation between the amounts of electrolyte and weight reduction ratios. It is clearly observed that the weight reduction ratio is a function of the initial amounts of electrolyte. Although there are some deviations, the relationship can be expressed with a linear equation as y = 0.13x − 0.08, where y is the electrolyte amount (g) and x is the reduction ratio (%). Therefore, the relation can provide remaining electrolyte amount in the cell using the reduction ratio.
Fig. 8 shows the total polarisation resistance of each cell with various amounts of electrolyte. It decreases rapidly from 38 to 51 % PFR, which is just a 0.5 g difference, indicating that the cell is very dependent on the electrolyte amount. From 38 to 51 % of PFR, it shows rather stable polarisation resistance. This means that those amounts of electrolyte are in the optimum range. At PFR 77 %, the polarisation resistance is minimal. As the electrolyte amount is close to the PFR 102 %, total polarisation resistance value increases rapidly again. This clearly shows that cell performance significantly depends on the electrolyte amount and optimum range of PFR existing. At the edge of the optimum range, the cell is very critical to the electrolyte amount. Consequently, a coin type MCFC needs an appropriate amount of electrolyte and permissible range of electrolyte is very narrow.
4. Conclusions
The effect of electrolyte amount on cell performance was investigated using a coin-type MCFC. Li/K carbonate was used as electrolyte, and four of initial electrolyte loadings of 1.5, 2.0, 3.0 and 4.0 g and total 16 cells were used. Then, the electrochemical performance was investigated at the loading and remaining electrolyte was analysed by the chemical way of weight reduction ratio into the 10 wt% acetic acid solution and SEM and EDS methods after operation.
Electrochemical analysis revealed that the performance was affected by Ohmic loss and mass-transfer resistance which depended on the amount of electrolyte. By increasing it to PFR 77 %, Ohmic loss and overpotential decrease. It should be that the masstransfer resistance at the cathode and the anode decreases by enlarging the active surface area caused by the increasing electrolyte loading. However, the PFR 102 % shows severe voltage loss at higher current density, representing that significant mass-transfer resistance takes place at the electrodes. This may result from flooding phenomenon. Based on the electrochemical analysis, the electrolyte loading is significantly related to the active surface area. Thus, it can be concluded that a permissible range of electrolyte is very limited in the MCFC, and that cell performance strongly depends on the electrolyte amount in the cell.
SEM and EDS results showed the distribution of electrolyte and revealed the poor electrolyte distribution on the cathode at low PFR. On the other hand, the PFR 102 % showed that all cells had been filled in with the electrolyte. Consequently, the flooding at the electrodes took place and raised mass-transfer resistance.
In addition, weight reduction ratio of the electrolyte into the acetic acid solution revealed that the remaining electrolyte amount depended on the initial electrolyte loading and with a linear equation as y = 0.13x − 0.08. Thus the remaining electrolyte after operation can be estimated by the relation.
Acknowledgements
This research was supported by Cooperation of Industrial R&D Programs of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry and Energy, Republic of Korea (No. 20143010031830).
References
T. Kouichi, in: O. Tokio (Eds.), Energy carriers and conversion systems, Vol. 2 (2009).
Kouichi T.. In : Tokio O., ed. Energy carriers and conversion systems (2009). Vol. 2I. Rexed, Application for Molten Carbonate Fuel Cells, (2014).
Rexed I.. Application for Molten Carbonate Fuel Cells (2014).S. Rosellini, G. Schmitt and P. Fleischmann, Molten Carbonate Fuel Cell: a novel approach to powering large telecommunications facilities, (2003).
Rosellini S., Schmitt G., Fleischmann P.. Molten Carbonate Fuel Cell: a novel approach to powering large telecommunications facilities (2003).S. McPhail, L. Leto, M. Della Pietra, V. Cigolotti and A. Moreno, International status of molten carbonate fuel cells technology, (2015).
McPhail S., Leto L., Della Pietra M., Cigolotti V., Moreno A.. International status of molten carbonate fuel cells technology (2015).H.-J. Choi, J.-J. Lee, S.-H. Hyun and H.-C. Lim, Int. J. Hydrogen Energy, 2011, 36, 11048-11055.
Choi H.-J., Lee J.-J., Hyun S.-H., Lim H.-C.. Int. J. Hydrogen Energy 2011;36:11048–11055. 10.1016/j.ijhydene.2011.05.184.M. Bischoff, J. Power Sources, 2006, 154, 461-466.
Bischoff M.. J. Power Sources 2006;154:461–466. 10.1016/j.jpowsour.2005.10.027.E. Antolini, Appl. Energy, 2011, 88, 4274-4293.
Antolini E.. Appl. Energy 2011;88:4274–4293. 10.1016/j.apenergy.2011.07.009.C.-G. Lee, S.-Y. Lee, B.-H. Ryu, D.-H. Kim and H.-C. Lim, J. Korean Electrochem. Soc., 2010, 13, 34-39.
Lee C.-G., Lee S.-Y., Ryu B.-H., Kim D.-H., Lim H.-C.. J. Korean Electrochem. Soc. 2010;13:34–39. 10.5229/JKES.2010.13.1.034.C.-G. Lee and H.-C. Lim, J. Electrochem. Soc., 2005, 152, A219-A228.
Lee C.-G., Lim H.-C.. J. Electrochem. Soc. 2005;152:A219–A228. 10.1149/1.1833318.J. R. Selman and P. H. Hsieh, Int. J. Hydrogen Energy, 2012, 37, 19270-19279.
Selman J. R., Hsieh P. H.. Int. J. Hydrogen Energy 2012;37:19270–19279. 10.1016/j.ijhydene.2012.06.021.J. R. Selman and L. G. Marianowski, in molten salt chemistry, D. G. Lovering (Eds.), Plenum, New York, (1982).
Selman J. R., Marianowski L. G.. In : Lovering D. G., ed. in molten salt chemistry Plenum. New York: (1982).J. R. Selman, in Fuel cell systems, L. J. M. J. Blomen, M. N. Mugerwa (Eds.), Plenum, New York, (1993).
Selman J. R.. In : Blomen L. J. M. J., Mugerwa M. N., eds. in Fuel cell systems Plenum. New York: (1993).H. Morita, M. Kawase, Y. Mugikura and K. Asano, J. Power Sources, 2010, 195, 6988-6996.
Morita H., Kawase M., Mugikura Y., Asano K.. J. Power Sources 2010;195:6988–6996. 10.1016/j.jpowsour.2010.04.084.C.-G. Lee, H. Hur and M.-B. Song, J. Electrochem. Soc. 2011, 158, B410-B415.
Lee C.-G., Hur H., Song M.-B.. J. Electrochem. Soc. 2011;158:B410–B415. 10.1149/1.3544941.C.-G. Lee, J.-Y. Hwang, M. Oh, D.-H. Kim and H.-C. Lim, J. Power Source, 2008, 179, 467-473.
Lee C.-G., Hwang J.-Y., Oh M., Kim D.-H., Lim H.-C.. J. Power Source 2008;179:467–473. 10.1016/j.jpowsour.2007.12.125.J. H. Hirschenhofer, D. B. Stauffer, R. R. Engleman, M. G. Klett, Fuel Cell Handbook 4th Ed., (1998).
Hirschenhofer J. H., Stauffer D. B., Engleman R. R., Klett M. G.. Fuel Cell Handbook 4th Ed.th ed. (1998).C.-G. Lee, Molten Carbonate Fuel Cells, DOI: 10.1007/SpringerReference_226338, (2013).
Lee C.-G.. Molten Carbonate Fuel Cells (2013).C.-G. Lee, J. Electroanal. Chem., 2016, 776, 162-169.
Lee C.-G.. J. Electroanal. Chem. 2016;776:162–169. 10.1016/j.jelechem.2016.07.005.E. Fontes, C. Lagergren and D. Simonsson, J. Electroanal. Chem., 1997, 432, 121-128.
Fontes E., Lagergren C., Simonsson D.. J. Electroanal. Chem. 1997;432:121–128. 10.1016/S0022-0728(97)00231-3.