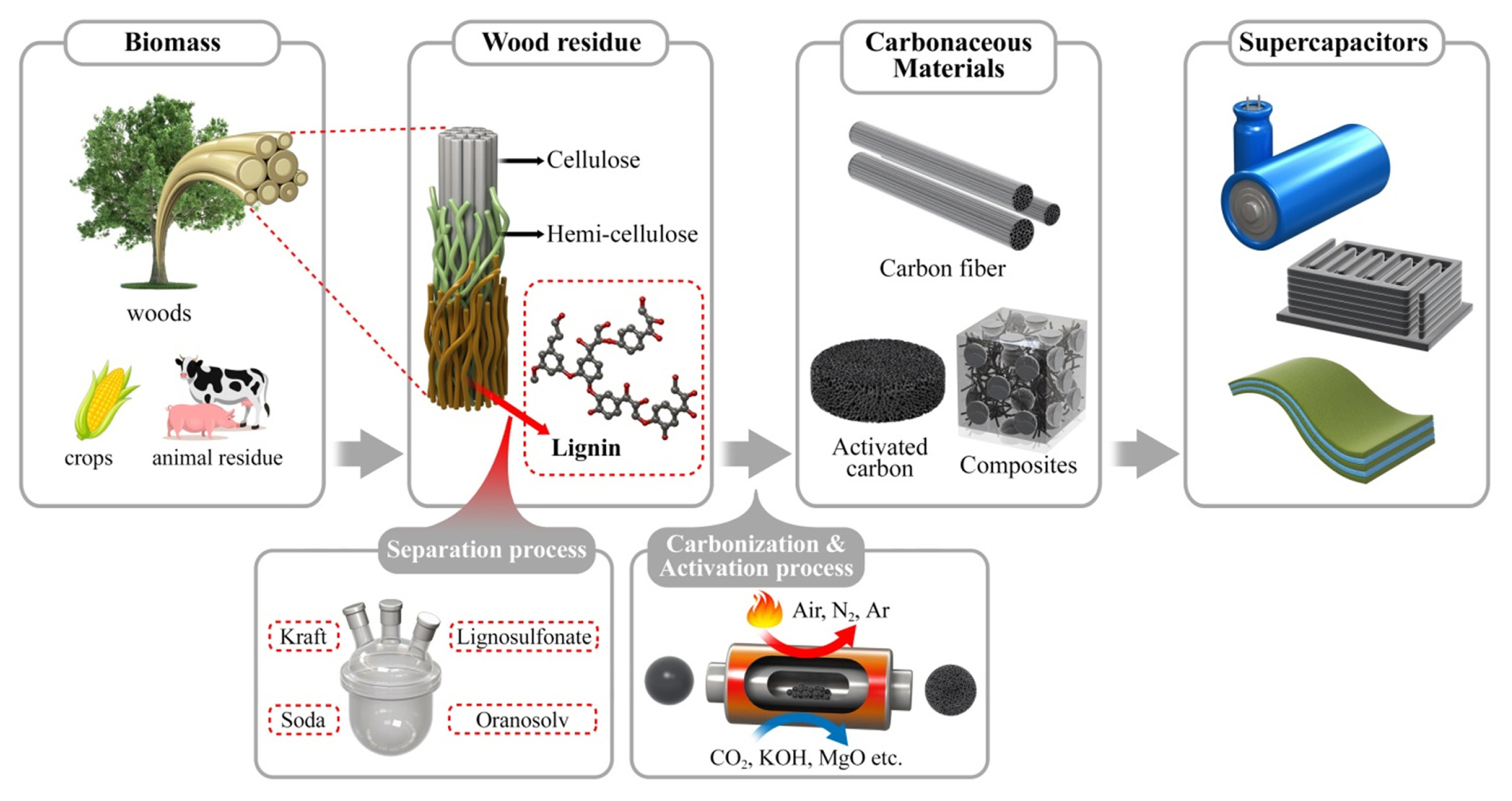
Recent Advances in Preparation and Supercapacitor Applications of Lignin-Derived Porous Carbon: A Review
Article information
Abstract
Lignin-derived porous carbon has been identified as a versatile electrode material for supercapacitors (SCs) in energy storage systems (ESSs) owing to their intrinsic advantages including good electrical conductivity, low cost, high thermal and chemical stability, and high porosity, which stem from high surface, appropriate pore distribution, tailored morphologies, heterostructures, and diverse derivates. In this review, to provide a fundamental understanding of the properties of lignin, we first summarize the origin, historical development, and basic physicochemical properties. Next, we describe essential strategies for the preparation of lignin-derived porous carbon electrode materials and then highlight the latest advances in the utilization of lignin-derived porous carbon materials as advanced electrode materials. Finally, we provide some of our own insights into the major challenges and prospective research directions of lignin-derived porous carbon materials for supercapacitors. We believe that this review will provide general guidance for the design of next-generation electrode materials for supercapacitors.
1. Introduction
In light of the rapid depletion of fossil fuels and the environmental issues with greenhouse gas emissions, a fast transition to renewable and sustainable energy sources from the past decades has been developed to spur our daily life with an efficient supply and energy realities [1,2]. In addition, the increasing recognition of the impacts of climate change, escalating energy costs and highly desire for environmentally friendly, renewable, and sustainable energy resources are globally fueling in the researches on biomass resources and their waste utilization [2–5]. Along the line, natural-inspired biomass materials would be the key to achieving environmental sustainability and energy realities, requiring research that is both disciplinary and interdisciplinary as well as energy saving with an efficient supply [2–4]. Generally, biomass is denoted as renewable plant/animal-based organic matters, which is used as fuel to produce carbohydrates via a conversion process, creating the building blocks of biomass [6]. As an alternative to fossil fuels, biomass materials are widely available and affordable carbonaceous material precursor with have a great potential because they can be used as a backup energy source for the electricity grid in case of supply disruptions due to the weather-dependent intermittency of other energy sources that are sustainable, such as solar, wind, and wave power, etc. [7,8].
Among biomass materials, wood-derived lignin-based materials have recently received extensive attention in diverse fields, including bioengineering, flexible electronics, and energy storage devices, for their own versatile utilization and application in preparation of functional materials [9–13]. For example, Qui et al. introduced lignosulfonic acid, a lignin-derived polymer, which has potent antiviral activity against human immunodeficiency virus (HIV) and herpes simplex virus (HSV) by blocking viral entry and sites [9]. Li et al. exploited a carboxymethyl chitosan/polyvinyl alcohol/cellulose nanofiber (CMC/PVA/CNF) matrix as lignin-based carbon to fabricate a flexible conductive hydrogel [12]. Li et al. developed a three-dimensional (3D) hierarchical porous carbon monolith electrode originated from lignin for SCs by combining soft- and hard-template method [10]. Park et al. reported all-lignin based flexible SCs by fabricating electrospun lignin/PAN carbon fibers with chemically cross-linked lignin hydrogel electrolyte [14]. Recently, Yeon et al. presented N-incorporated honeycomb-like nanoporus carbons via hydrothermal carbonization of a lignin precursor for lithium-sulfur battery cathode [15].
Lignin is one of the primary components (cellulose, hemicelluloses, and lignin) in lignocellulosic biomass. Also, it is the most abundant bio-polymers, which is constituted of 30% of non-fossil organic carbon on Earth, and 20 to 35% of the dry mass of wood as a byproduct of pulping and biorefinery [16–18]. Moreover, it stands as one of the most abundant sources of naturally produced aromatic polymers in wood [2,19,20]. Because of its aromatic nature, lignin has a considerable promise as a precursor/raw material for the use of application of bulk or modified aromatic compounds, thus providing suitable alternatives to commercial values of petroleum-derived BTX compounds (benzene, toluene, and xylene) [2,19,21], as well as a higher energy density than polysaccharide polymers [20]. However, the structural complexity of lignin hinders the precise determination of its heterostructures and functionalities [2,21]. Moreover, merely around 2% of the total production of lignin isolated from pulping process is employed to obtain high-value products, while the remaining portion is combusted for heat and energy or discarded due to limited low economic values [16,17,19]. This underutilization drives the need to develop processes for more efficiently separation of lignin from biomass as well as for the fabrication lignin-derived carbonaceous materials with higher economic value.
Despite the structural complexity of lignin and its derivates, their high contents of carbon, low cost, and abundant sources render them suitable as precursors of electrode materials for energy storage applications, such as SCs and lithium ion batteries (LiBs) [11,22–24]. Moreover, lignin has been recently employed to prepare redox-active materials and electrocatalysts despite being chemically unstable and electrically insulating [25,26] because it contains phenolic hydroxyl (or methoxy) groups that are easily transformed into redox-active quinone/hydroquinone (Q/QH2) moieties [27]. Therefore, it is essential to explore lignin-based carbon materials as new electrode materials for SCs to address the alternative energy resources.
In this mini review, we present a comprehensive and up-to-date progress in preparation and utilization of lignin-derived porous carbon as electrode materials for SC applications. First, we briefly describe the origin and fundamental physicochemical properties of lignin, and then highlight some of the latest advances in the application of lignin-derived porous carbon electrode materials for SCs. Finally, this mini-review concludes by offering our own insights into the key challenges and potential solutions, aiming to guide future works on lignin-based electrode materials for SC applications.
2. Lignin
Historical development of lignin and its physicochemical properties
Lignin was originally mentioned in 1813 by the Swiss botanist A. P. de Candolle, who characterized it as a fibrous, flavorless substance that is soluble in weak alkaline solutions but insoluble in water (H2O) and alcohol and precipitates from solution using acid [18,28]. The word “lignine” is derived from the Latin term “lignum”, which means wood [28]. Later, Anselme Payen, a French chemist, found the when wood was treated with nitric acid and alkaline solutions, “cellulose” resulted in a significant insoluble residue in 1838, while dissolved incrustants were later called “lignin” by Frank Schulze in 1865 [29]. In 1866, E. Erdmann initially proposed that the “glycolignose” wood included covalent bonds between lignin and carbohydrates [30]. Inspired by the pioneering work of Erdmann, other functional groups, such as methoxyl lignin, in a large volume in a cell wall of lignocellulostic materials, such as woods, were investigated by Benedikt and Bamberger in 1890 [31]. Subsequently, the lignin structure and chemistry were thoroughly investigated in the academic and industrial fields. For example, Carl F. Dahl, who is a German chemist, patented and developed the pulping process by employing the hot solution of sodium hydroxide and sodium sulfide to produce pulp by breaking bonds between lignin, hemicellulose, and cellulose in wood in 1884, which was later called as “Kraft pulping” [32]. Then, P. Klason reported the macromolecular structure and the element composition of lignin in 1908 and 1920, respectively [33–35]. In the past decades, tremendous research efforts have been devoted to the elucidation of the structure of lignin and development new methods for its isolation/separation.
Numerous works have proved that lignin is an amorphous aromatic polymer with a 3D matrix made up of methoxylated phenylpropane units connected by ether linkage and C–C bonds [2,21,36]. Commonly, lignin is composed of three types of structural sub-units, namely guaiacyl unit (G), p-hydrophenyl unit (H) and syringyl unit (S), which are formed via combinatorial radical coupling from the three main monolignols (p-coumaryl, coinferyl, and sinapyl alcohols) [36]. Lignin contains a variety of linkages, and their type and ratio depends on the plant source [24]. Typical structures of lignin and the most common linkages are shown in Fig. 1. Generally, the β-O-4 ether linkage (C–O bond) accounts for around 50% of all links on average and others are found in a series of C–C linkages (β-β, β-5, β-1, etc.) [36,37]. Further, the physicochemical properties of lignin depend on the monolignol contents in plants. Since lignin is randomly fragmented into smaller units during the separation process, the molecular mass of lignin isolated from plants typically ranges from 1000 to 2000 g mol−1, which hinders determining the precise molecular mass [22]. The glass-transition temperature (Tg) of lignin ranges from 170 to 190°C depending on the level of crosslinking and crystallinity, which is influenced by the quantity of H2O and polysaccharides, molecular weight, and chemical functionalization [22,36,38]. Because the degree of condensation has the greatest impact on Tg, lignin with higher molecular weight exhibit higher Tg [22]. Therefore, the structural and physicochemical properties of lignin vary with the chemical structure, molecular weight, S/G ratio and molecular behavior, which can be tuned using diverse separation processes [38,39].

(a) The schematic topical focus of the current review contents. Adapted from Ref. [11] with permission from American Chemical Society. (b) Monomeric precursors of lignin, H, G, and S structural units, and a lignin structure connected the most common inter-unit linkages. R = lignin. Adapted from Ref. [17] with permission from WILEY-VCH Verlag GmbH & Co.
3. Preparation of lignin-derived carbonaceous materials
3.1. Classification of lignin depending on the process of extraction/separation from wood
Numerous methods have been utilized to extract and modify lignin as a byproduct of paper pulping industry and biorefinery. The efficient extraction or isolation of lignin with high purity and a less condensed structure from lignocellulose is essential for the valorization of lignin [40,41]. Lignin, formed through a radical mechanism, is challenging to extract due to its covalent linkage with hemicelluloses, forming a lignin-carbohydrate compounds [42]. Breaking the bonds of lignin-carbohydrate compounds and partially depolymerizing lignin are necessary for its extraction. This depolymerization involves breaking down lignin into smaller fractions, facilitating its solubilization from biomass. The high reactivity of lignin and its fractions often leads to repolymerization (condensation) reactions, resulting in the formation of new C–C bonds during lignin extraction [42]. Consequently, different lignin intermediates with diverse properties are generated. The structure of isolated lignin generally varies due to differences in feedstocks, extraction methods, and extraction severity. Generally, lignin can be classified into four categories according to its functional groups and separation process: Kraft lignin (KL), Lignosulfonate lignin (LS), Soda lignin (SL), and Organosolv lignin (OL).
3.1.1. Kraft lignin (KL)
The kraft process is one of most common process for pulping industry [2]. A solution containing sodium hydroxide (NaOH) and sodium sulfide (Na2S) is used in alkaline medium at a relatively high temperature (140–170°C) to extract lignin from lignocellulosic materials [37]. The lignin acquired through this process possesses a sulfur content ranging from 1.5% to 3% by weight. It is soluble at a pH greater than 10 and demonstrates a high level of purity [43]. Moreover, it contains some aliphatic thiol groups, which give it a characteristic odour, noticeable especially during heat treatment [44]. As a high concentration of C–C bonds and strong ether bonds are cleaved during the delignification and acidification of lignin, phenolic hydroxyl groups form due to the breaking of aryl bonds [31,38]. The phenolic groups on lignin are ionized during the treatment in an alkaline environment, leading to the formation of a black pulp soluble in H2O [38]. Kraft lignin exhibits hydrophobic properties, necessitating modification to enhance its reactivity [45].
3.1.2. Lignosulfonate lignin (LS)
Lignosulfonate lignin involves the use of sulfuric acid and/or a sulfite containing magnesium, calcium, sodium, or ammonium to extract the lignin from woods in a wide pH ranges during the sulfite pulping process [28,46]. During sulfite pulping, lignin undergoes chemical modification via sulfonation, leading to the incorporation of anionic groups, while biomass is heated in a sulfite medium at a low or neutral pH [46,47]. Lignosulfonate lignin is characterized by a highly cross-linked structure, with a sulfur content of around 5%. It features two types of ionizing groups: sulfonic and phenolic hydroxy groups [48]. Lignosulfonate lignins are soluble in H2O, high molecular weight, higher ash content than kraft lignin, about 4–8% [42,47]. Moreover, lignosulfonates are appropriate for a range of potential applications, such as colloidal suspensions, stabilizers, dispersants, binders, detergents, and adhesives, due to the high concentration of active sulfonic acid groups [49].
3.1.3. Soda lignin (SL)
Interestingly, soda lignin is formed in the delignification process, which is related to the kraft pulping process in terms of the processing conditions and principles without sulfur compounds [49]. Another distinguishing feature is the existence of vinyl ethers [50]. This approach is primarily utilized for the production of lignin from annual plants and plant waste, which typically have lower lignin content [44]. Soda lignin, resulting from this method, possesses higher purity and a lower molecular weight range of 800–3000 g mol−1 compared to kraft lignin (1500–5000 g mol−1) [51]. Additionally, soda lignin demonstrates high silicate and molecular nitrogen content [49]. Interestingly, lignin is solubilized by heating in the presence of chemicals such as the sodium hydroxide (NaOH)-anthraquinone [52]. Soda lignin is generally used for non-woody fibers, such as grass, straw, and sugarcane bagasse [38]. The soda method significantly promotes β-aryl ether bond cleavage and inhibits condensation of the resulting lignin segments when the anthraquinone/anthrahydroquinone redox reaction undergoes [52]. Due to the oxidation of the aliphatic hydroxyl groups, soda lignin possesses a relatively higher concentration of carboxylic acid groups [45].
3.1.4. Organosolv lignin (OL)
In the organosolv process, lignin is separated from the lignocellulosic biomass via solubilization with organic solvents [28,31]. This process is more environmentally friendly than kraft or sulfite process because it is sulfur-free and nontoxicity [38]. Organosolv lignin is characterized by a homogeneous structure and its low molecular weight, ranging from 500 to 5000 g mol−1 [51]. It is soluble not only in alkaline systems but also in a wide range of organic polar solvents [48]. Some studies indicate that heightened severity in organosolv processes results in a significant decrease in the molar mass of the extracted lignin, ranging from 36% to 56% compared to untreated lignin. Furthermore, this process leads to a reduction in aliphatic hydroxyl group content, an increase in syringyl phenolic units, and the formation of condensed phenolic structures [53]. Due to its environmental benefits, organosolv lignin has more attention as a pretreatment for biofuel production even though it is not often employed in pulping industry [52]. Organosolv lignin exhibits strong solubility in organic solvents and nearly insoluble in water due to its significant hydrophobic nature [31]. Compared to other technical lignins, organosolv lignin is more soluble in organic solvents and has lower sulfur content, higher purity, a narrow molecular-weight range, and diminished molecular weight [54].
3.2. Preparation of electrode materials using lignin-derived porous carbon
As a pure carbon precursor, lignin can be easily converted into high-purity porous carbon materials by etching process. Several synthetic techniques can be applied to turn lignin-derived carbon into porous functionalized carbon materials after carbonization. In the synthesis of electrode materials, the conventional carbonization-activation methods are extensively utilized in the preparation of lignin-based porous carbon materials. A schematic illustration of preparation process of lignin-derived porous carbon electrodes is shown in Fig. 2.
3.2.1. Physical activation
Physical activation is referred to as the step (carbonization-activation) process of thermal activation, which involves the carbonization of carbonaceous raw materials with gaseous (CO2 and air), steam or mixture of gaseous and steam at a temperature between 400 and 850°C [55,56]. For activation process, the temperature usually ranges between 600 and 900°C [57]. To overcome the sluggish reaction rate at temperature around 800°C, CO2 is commonly used as an activating agent because it is clean and simple to handle [55,56]. However, air activation is difficult to manage the rate of activation. The process of air activation at low-temperature (<600°C) generally produces CO2, whereas carbon monoxide (CO) is more easily obtained at high-temperature air activation (>900°C) [58]. Also, the carbonization process releases CO2, CO, H2O, and several small-molecule volatile substances, leading to the formation and disappearance of certain pores in the lignin-char with the shrinkage effect at a high-temperature process [58]. According to Aworn et al., the precursor materials and activation techniques can also result in a variety of activated carbons to achieve high specific areas with distinct properties [59]. For example, Saha et al. utilized a simple physical (CO2) and chemical activation (KOH) to produce activated mesoporous carbons using Plurounic F127 surfactant [60]. The activated porous carbons displayed 1.5- to 6-fold increases in porosity with a high SSA of 624 m2 g−1 and a pore volume of 0.73 cm3 g−1 during physical activation process. In another work, Jayawickramage et al. fabricated carbon nanofiber mats derived from polyacrylonitrile (PAN)-lignin blends via electrospinning followered by CO2 activation process with a high SSA of 2730 m2 g−1 [61]. In general, physical activation aims to establish internal pore structures within the carbon matrix while effectively maintaining the generated pores during the thermal process through an appropriate condition of the starting materials and carbonization techniques.
3.2.2. Chemical activation
Chemical activation, which involves vigorous etching of KOH, K2CO3, or ZnCl2 at high temperatures, is a widely employed method to produce lignin-based porous carbon materials with high specific surface area (SSA) [57]. Chemical activation is a step procedure with simultaneously employing the carbonization and activation process [55]. In the KOH activation process, which produces porous carbon materials with high SSA range from 1000 to 3000 m2 g−1 [58], the carbon framework is etched through the redox reaction occurring between diverse potassium compounds as chemical activation agents in conjunction with the carbon matrix. In the activation process, the dehydration of KOH forms K2O and water. Then, the decomposition of K2CO3 forms K2O, which results in the formation of gaseous byproducts of CO2. CO2 and H2O, also trigger the temporary physical carbonization of the carbon matrix [58]. The activation reaction can be simply described as following.
The K2CO3 and ZnCl2 are also widely used for activation agents because their dehydration effect facilitates the dissolution of lignin at relatively low temperature. [58]. Equations (6)–(8) show the chemical reaction mechanism by ZnCl2.
Generally, the pyrolysis of wood lignins without a catalyst showed the primary evolution range for CO2 occurring between 400 and 600°C [62]. It is noted that ZnCl2 has a melting point of 283°C and a boiling point of 732°C [63]. It has been determined that the evolution observed between 200°C and 300°C can primarily be ascribed to the release of moisture, closely linked with the solid-phase ZnCl2 [63,64]. The evolution occurring between 400 and 800°C is believed to be a result of the release of volatiles and the gradual evaporation of the liquid-phase ZnCl2 [65].
Finally, porous carbon can be obtained by washing off the ions using an acid and/or an alkali, depending on the chemical agents employed in process [56]. As a result, the porosity of the activated carbons essentially originates from the dissolution of chemical components in carbon structures. For example, Zhang et al. prepared lignin-derived hierarchical porous carbon via KOH activation with a high BET specific surface area (SSA) of 3775 m2 g−1 [66]. Recently, Lim et al. compared biomass raw materials and lignin extracted by different methods (Klason, organosolv methods) with activated carbon prepared by KOH activation and made into supercapacitors. The gravimetric specific capacitance of biomass raw material, Klason lignin, and Organosolv lignin were 129, 131, and 130 F g−1, respectively. This indicates that even by utilizing only lignin, it was possible to achieve better performance than using raw materials, demonstrating the efficient utilization of lignin [67]. In another work, Wu et al. introduced a simple and easy way to develop lignin-based activated carbons with different activating agents (ZnCl2, KOH, K2CO3) for electrode materials in supercapacitor application [68]. The BET surface areas of the asprepared carbon electrodes were measured to be 866, 1191, and 1585 m2 g−1, respectively.
3.2.3. Template method
Template method is an effective approach for the synthesis of porous carbon with tunable pore size and porosity [58,69], in which templates are used as the porogen for generating pores in the carbonization process. Generally, the template includes hard template and soft template. The hard templates, such as MgO [70], ZnO [71], SiO2 [72], and ZnC2O4 [73], are filled with carbon precursor, which serve as templates for the replication of carbon, where carbonization occurs with the pores [74]. Hence, the well-defined template structure predetermines the morphology of the resultant carbons [75]. In contrast, soft templates utilize a variety of block copolymers and co-assembled materials, such as Pluronic P123 [76] and Pluronic F127 [60], which exhibit an ordered scaffold. Moreover, soft template can be easily decomposed either completely or partially during the carbonization process, which leading to the formation of internal pores within the carbon matrix [58]. Thus, template-assisted porous carbon materials depend on both the attributes of the template and the specific steps involved in the self-assembly process. For example, Song et al. synthesized an unique mesoporous carbon from lignin, employing a nano-MgO template with Pluronic F127 [70]. As-prepared electrode displayed the BET SSA of 712 m2 g−1 and total pore volume of 0.9 cm3 g−1, respectively. In another work, Fu et al. devised a facile approach to synthesize of lignin-derived porous carbons, utilizing gasexfoliation aided by zinc oxalate and in-situ templating, resulting in a two-dimensional quasi-nanosheet architecture [73]. While template-assisted methods are well-established processes for synthesizing porous carbon materials, it still remains a challenge to achieve lignin-derived porous carbons with adjustable micro/mesopores and high SSAs.
4. Fabrication of electrode materials for SCs
Up to now, the improvement of preparing novel electrode materials with high energy and power density, which could be summed up through materials and/or method innovations, plays an essential role in supercapacitor applications [22,23,58]. Here, we elaborate on several suitable strategies for preparing electrode materials in the following section. Also, the recent advances in lignin-derived porous carbon electrode materials are summarized in Table 1.
4.1. Carbon fibers
Carbon fiber has an extensive use in various engineering applications and has been utilized as a reinforcing agent in polymer-matrix composites, linked in automotive, aerospace, construction, and energy industries due to its exceptional tensile strength and high Young’s modulus [77]. Carbon fiber can be derived from renewable plant fibers, such as bamboo and hemp, which offer low density, high specific strength, and eco-friendly properties [24]. For the first preparation of carbon fiber (CFs) from commercial lignin, Kadla et al. reported the preparation of carbon fiber from various commercial lignins via a thermal extrusion without any chemical modification [78]. In their work, polyethylene oxide (PEO) used as a precursor to produce fiber with kraft hardwood lignin in order to improve thermal stability and fiber spinning performance during following carbonization. Owing to their low density, high strength, and high conductivity, lignin-derived carbon fibers have attracted attention as potential electrode materials for energy storage and conversion devices, such as SCs and LiBs [22,23]. However, the preparation of high-performance carbon fibers from pure lignin has several limitations due to the inherent characteristics of lignin, including its low molecular weight, wide distribution of molecular sizes and complex heterostructures [24]. To solve these drawbacks, several effective approaches have been developed to synthesize high performance carbon fibers using polymers as precursors [39], such as polyacrylonitrile (PAN), cellulose-acetate (CA) and polyvinyl alcohol (PVA). Among them, PAN is the most widely employed CF precursor material due to its notable mechanical strength and significant carbon content, particularly in the context of electrospinning for electrode fabrication [79]. For example, Zhu et al. introduced a simple modification and fractionation approach to prepare high-performance lignin-based carbon fibers [79]. They found that the modification and fractionation procedure result in increasing the molecular weight and lowering the heterogeneity of lignin. The addition of lignin with a high molecular weight and low heterogeneity facilitate to preserve the morphology of lignin-based CFs, which consequently results in a significant increase in the specific surface area and energy storage capabilities. The specific surface area and specific capacitance of the prepared lignin-based CFs showed 2042.86 m2 g−1 and 442.2 F g−1, respectively. Recently, Dai et al. exploited nitrogen oxygen co-doped esterified lignin/polyacrylonitrile (PAN) based CFs [80]. They used lignin as carbon precursor and O source as well as PAN as auxiliaries and N source. They found that esterification of lignin transforms the hydroxide radical to butyl esters, resulting in reducing glass transition temperature (Tg) of lignin, which contributes to the formation of a bonding structure between fibers during thermostabilization process. The as-prepared electrode displayed an extremely high specific capacitance, measuring 320 F g−1 at 1 A g−1 and 200.4 F g−1 at 20 A g−1 in 6 M KOH electrolyte. Moreover, the assembled symmetric supercapacitor cells achieved an outstanding energy density of 17.92 Wh kg−1 at power density of 800 W kg−1 as well as an impressive cycling stability, maintaining 94.5% retention after 5000 cycles.
Considering the hydrophobicity nature, limited malleability, and low oxygen content, CA provides a series of advantages as a versatile polymer that possesses hydrophilic properties, exceptional flexibility, and a significant oxygen content [81]. For example, Cao et al. employed a simple phosphating technique to alter CA and lignin, resulting in a precursor materials suitable for energy storage applications [82]. Fig. 3a illustrates the preparation process of the phosphoric acid-functionalized carbon fiber, resulting in good thermal stability and flexibility of lignin-derived materials. Significantly, they found that the esterification process involving phosphoric acid (H3PO4) effectively reduce the hydrogen bond interactions between lignin molecules. As a result, the flexibility and spinnability of the spinning solution improve as the content of H3PO4 increases. This improvement is attributed to a noticeable reduction in the occurrence of “bead” defects in the solution, as shown in Fig. 3b–d. The electrode revealed a notable specific capacitance of 346.6 F g−1 when tested at current density of 0.1 A g−1. Moreover, the assembled supercapacitor cell delivered substantial energy density of 31.5 Wh kg−1 at a power density of 400 W kg−1. Remarkably, even when operating at the highest power density of 4000 W kg−1, the supercapacitor still remained an energy density of 24.3 W kg−1.
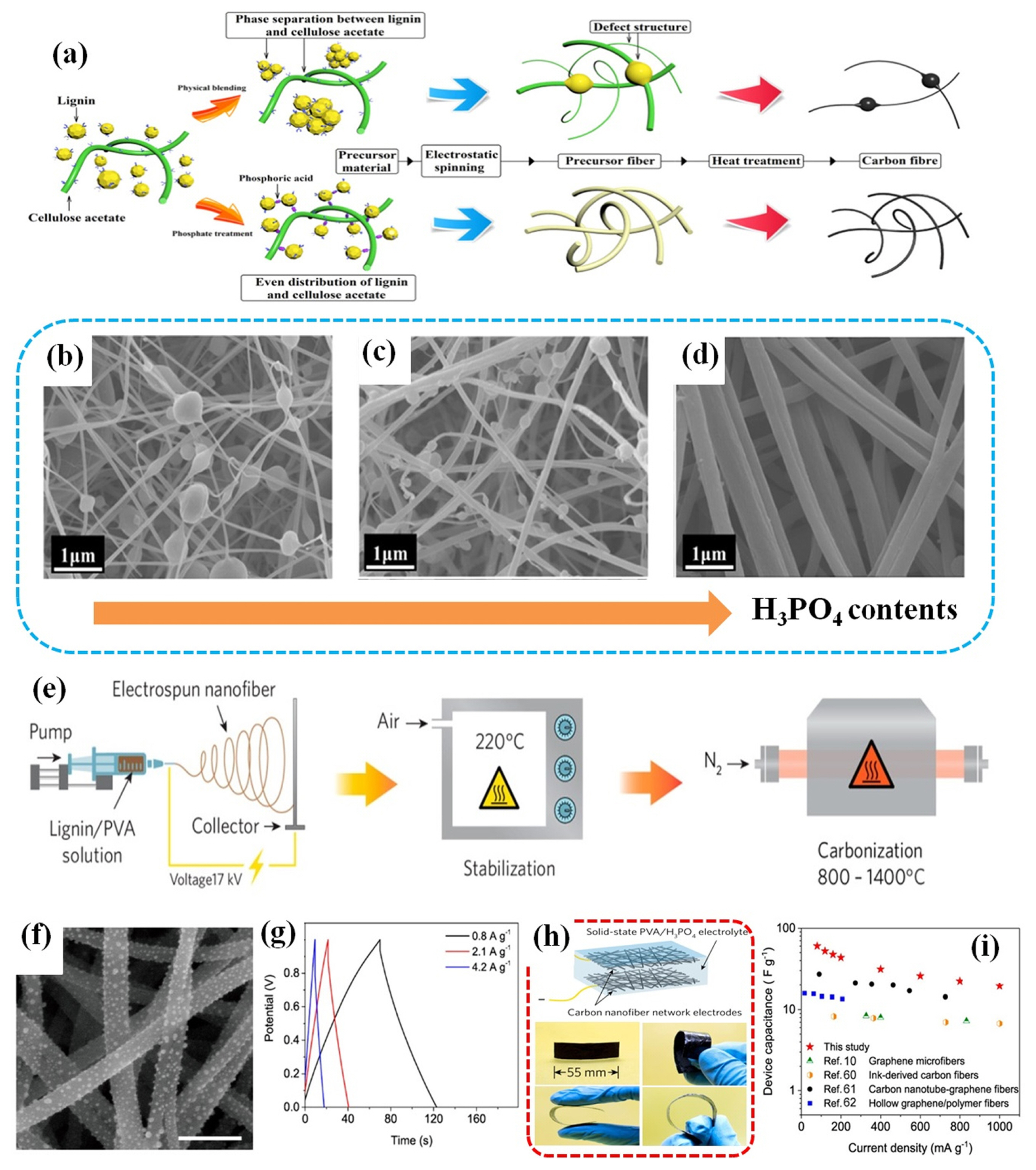
(a) Schematic illustration of phosphoric acid-functionalized carbon fiber. SEM images of (b) PFs-1 (the mass ratio of H3PO4/lignin:0/100), (c) PFs-3 (the mass ratio of H3PO4/lignin:20/100), (d) PFs-5 (the mass ratio of H3PO4/lignin:40/100). Adapted from Ref. [82] with permission from American Chemical Society. (e) Schematic illustration of the synthesis processes of the carbon nanofiber networks. (f) Magnified SEM image of C800 showing the dot-like features on the surface with scale bar of 500 nm. (g) Galvanostatic charge-discharge curves of the SC-L with C1000 electrode under different current densities. (h) Photographs of the thin and free-standing FS1000 illustrating its flexibility. (i) Device capacitance of FS1000 at different current densities compared to other all-solid-state supercapacitors reported in the literature. Adapted from Ref. [83] with permission from American Chemical Society.
PVA is also commonly mixed with lignin for the preparation of CFs because its water-soluble and biocompatible PVA in H2O allow avoiding the use of toxic solvent during processing [39]. For example, Wei et al. established a unique freestanding and flexible carbon nanofiber networks through systematical analysis on the effect of different carbonization temperature by a simple mixing alkali lignin with 5 wt% PVA aqueous solution (lignin/PVA, 75/25, w/w) [83], illustrating synthetic procedure in Fig. 3e. The asobtained electrode reached excellent specific gravimetric capacitance of 240 F g−1. It was noted that some roughness with dot-like features was shown on the nanofiber surface, as displayed in Fig. 3f. This could be attributed to the existence of inorganic moieties within the lignin. Interestingly, they developed an all-solid-state supercapacitor device utilizing carbon nanofiber networks derived from lignin. These networks possess favorable mechanical properties, enabling their incorporation into potential wearable electronics, as shown in Fig. 3h. The as-prepared electrode achieved a specific capacitance of 60.4 F g−1 in device, resulting in a gravimetric energy density of 8.4 Wh kg−1 (Fig. 3i).
4.2. Activated carbon
Activated carbon has been widely utilized in the design and fabrication of supercapacitor electrode materials because of its advantages, which encompass a sizable specific surface area, favorable electrical conductivity, economical cost, impressive thermal and chemical stability, and high porosity [84]. Also, activated carbon is generally synthesized by carbonization process of carbon precursor followed by activation process [55]. As mentioned above, there are three main types of preparation method for porous carbon electrode materials; physical activation, chemical activation, and template method have been developed for the preparation of lignin-based porous carbon materials.
Generally, physical activation is referred to as gaseous activation [85]. Steam, CO2, and O2 are widely used during the physical activation process [58,86]. During the process, small and well-developed pores can merge on the surface and inside of the carbonized materials by physical etching of active agents (steam, CO2 and O2), resulting from the gas escaping in activation reaction [58]. Researchers have shown that physical activation offers designable pore size distribution with more accuracy and narrowness, resulting in more micropores [87,88]. For example, Saha et al. reported activated mesoporous carbons from precrosslinked lignin via Plurounic F127 surfactant with the enhanced meso- and microporocity after physical (CO2) and chemical activation (KOH) [60]. The assynthesized mesoporous carbon electrodes showed high SSA of 624 and 1148 m2 g−1, respectively. Also, the physical- and chemical-activated porous carbon electrodes with mesoporosity of nearly 79 and 66% from lignin exhibited a capacitance of 102.3 F g−1 and 91.7 F g−1 in 6 M KOH electrolyte, respectively. Apparently, it was noted that both physical and chemical activation improved the electrochemical performance of the electrodes with meso- and microporosity. In another work, Jayawickramage et al. fabricated binder free carbon nanofiber derived from polyacrylonitrile-lignin blends via electrospining followered by CO2 activation process [61]. They firstly found that the electrode derived from PAN:ligin ratio of 70:30 exhibited excellent physicochemical properties, such as high specific surface area (SSA: 2730 m2 g−1), high mesoporosity, and high electrical conductivity, as well as good specific capacitance of 128 F g−1. Moreover, when utilizing an ionic liquid electrolyte and operating at 3.5 V, the assembled coin-cell supercapacitors were capable of providing an energy density of 59 Wh kg−1 along with a power density of 15 kW kg−1. Recently, Park et al. investigated hierarchically structured phosphorus-incorporating steam-activated lignin-based nanoporous carbons (P-aCNs) for pseudocapacitive Na+-ion storage device with remarkably improved rate and cyclic capabilities in organic electrolyte [89]. Importantly, they prepared hierarchically P-incorporating nanoporous carbon via a hydrothermal method without any separation or chemical regents. In their procedure, lignin underwent transformation, leading to the formation of numerous oxygen functional groups through condensation and polymerization, ultimately resulting in the precipitation of a solid product. Remarkably, P-aCNs electrode displayed exceptional cyclic stability, with a 96.0% retention over 100,000 cycles in a full cell setup, along with a high capacitance of 265.43 F g−1 and a rate capability of 75%. They believed that the significant pseudocapacitive characteristics of P-containing groups in organic Na-ion electrolytes are affirmed through surface area-independent and surface-restricted capacitances, distinct redox waves, and a strong affinity for Na-ions. Additionally, they concluded that these pseudocapacitive properties of P-aCNs in Na-ion systems are probably attributed to their interconnected porous structure and redoxactive P=O bonds through both experimental and computational analyses.
Hydrothermal methods enable the transformation of lignin into carbonaceous substances with elevated water content at relatively low temperatures [90]. In this process, lignin undergoes decomposition in a water medium, generating phenolics that subsequently polymerize to yield phenolic carbonaceous products during hydrothermal carbonization. These products typically exhibit a high concentration of oxygen functional groups and a low degree of aromatization, rendering them effective precursors for chemical activation [91]. The hydrothermal carbonization of lignin for the production of activated carbon derived from lignin generates reduced CO2 emissions compared to traditional carbonization methods and eliminates the need for protective gas flow [92]. The procedure involves subjecting lignin to hydrothermal treatment for carbonization, followed by mixing the resulting dried product with KOH to create lignin-based activated carbon. The oxygen content diminishes with increasing activation temperature and the KOH/lignin ratio. In an interesting work of Park’s group, they utilized a a separation-free, straightforward, and controllable one-pot hydrothermal approach to produce heteroatom-doped and undoped carbon dots (CDs) and nanoporous carbons (CNs) with improving the optical, electronic, and electrochemical properties [93]. They found that the P, N, and S-doped aCNs (donated as P-aCN, S-aCN, and N-aCN, respectively) display sturdy honeycomb, fully exfoliated reed-like, and individually hollow reed-like structures, with distinctive hierarchical porosity. Among them, it was interesting to find that N-aCN presented the highest CO2 adsorption capacity and exceptional regeneration potential due to the presence of essential N-containing functional groups and their role in maintaining structural integrity. In terms of the supercapacitive performance, P-aCN revealed the highest capacitance of 298.43 F g−1, whereas S-aCN displayed outstanding rate and cyclic capacibilities of 90.26 and 98.26%, respectively. It is considered that redoxactive P=O bonds lead to high pseudocapacitive behaviour, while distinctive porous structure and the presence of S-containing groups result in excellent rate and cyclic capabilities. More recently, Schlee et al. employed KL as a precursor to prepare porous carbon fiber mats via CO2 activation without any addictives. CO2 activation process allowed the electrode to possess mainly basic oxygen functional groups with high SSA of 1204 m2 g−1 as well as the enhanced the micro- and meso-porosity [94]. It was noted that the porosity of the CO2 activated fiber increased dramatically, as displayed in Fig. 4a (TEM analysis). The non-activated fiber was dense, while CO2 activated fiber appeared less dense. From the N2 and CO2 ad-/desorption measurement by density functional theory (DFT) models in Fig. 4b, the resulting pore size distribution revealed that non-activated fiber possessed large amount of ultra-micropores (< 1 nm), whereas CO2 activated fiber accommodated both meso (2–50 nm) and micro (< 2nm) pores, supporting increased porosity of the fiber. The as-prepared CO2-activated fiber mats revealed a notable specific gravimetric capacitance of 155 F g−1 at 0.1 A g−1. Additionally, they demonstrated impressive performance at different rate, retaining a capacitance of 113 F g−1 even at a high rate of 250 A g−1 in 6 M KOH electrolyte.
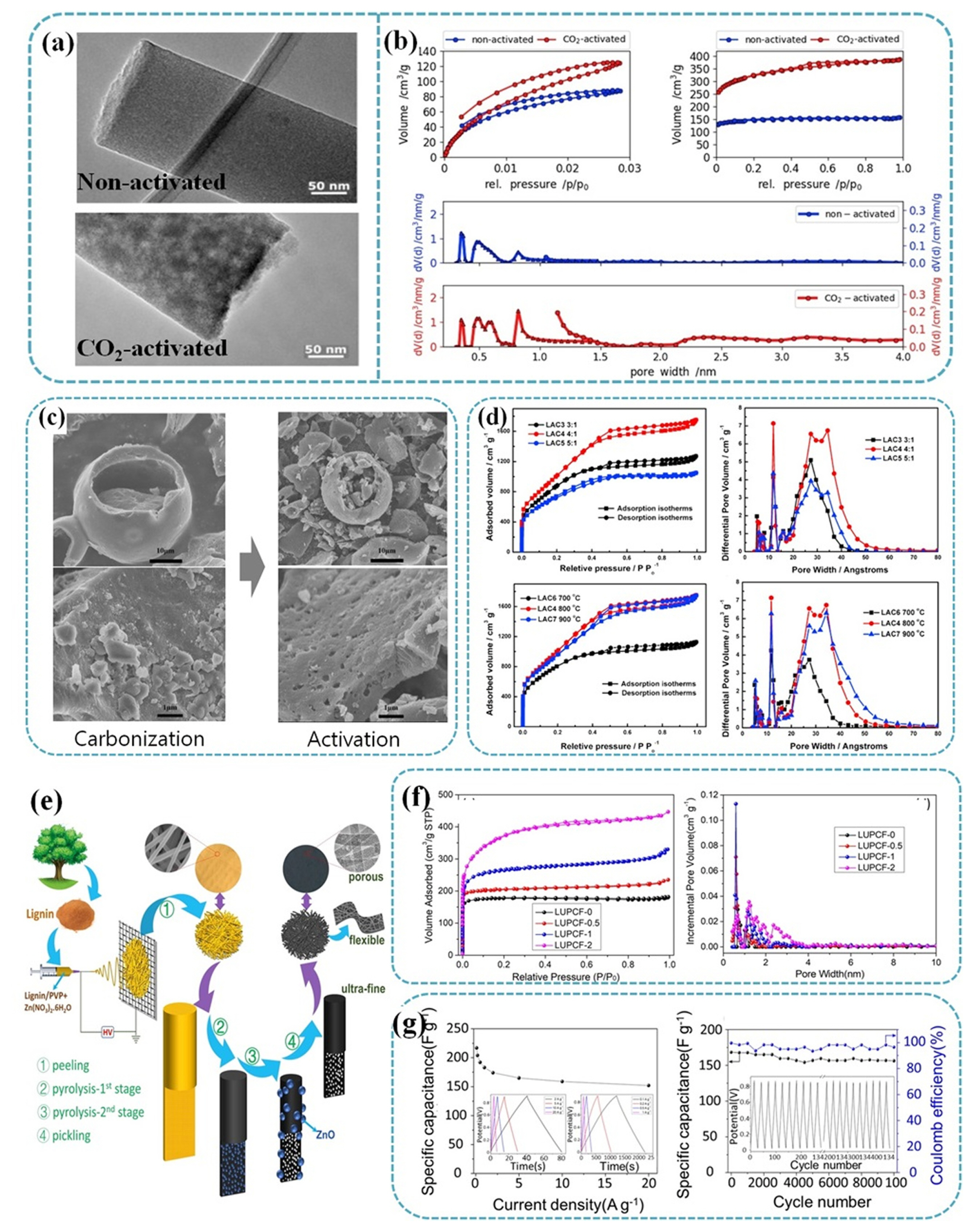
(a) TEM images of non-activated fiber and CO2-activated carbon fiber. (b) CO2 and N2 ad-/desorption isotherms of the non-activated (blue) and CO2-activated (red) carbon fiber mats with pore size distributions calculated by DFT based on the adsorption isotherms. Adapted from Ref. [94] with permission from Elsevier Ltd. (c) SEM images of the carbonized lignin samples at 500°C and the activated carbon LAC4. (d) N2 ad-/desorption isotherms and pore size distribution of LACs prepared from KOH activation in different KOH-LC ratio and different temperature. Adapted from Ref. [66] with permission from Elsevier B.V. (e) Schematic of the fabrication procedure of LUPCFs. (f) N2 ad-/desorption isotherms and pore size distribution of LUPCFs prepared different ratio of ZNH (0/0.5/1/2 g). (g) Electrochemical performance of LUPCF-2 in a two-electrode system showing specific capacitance at different current densities (Insets: GCD curves at different current densities) and cycling stability at current density of 5 A g−1. (Insets: GCD curves of the first 10 and last 10 cycles). Adapted from Ref. [71] with permission from Elsevier Inc.
Porous carbon materials with high SSA can also be prepared via chemical activation of carbon precursor. It is generally accepted that high SSA allows more active sites and easy access of electrolyte, resulting in enhanced electrochemical performance. For example, Zhang et al. investigated lignin-derived hierarchical porous carbon via traditional carbonization-activation method (KOH activation) [66]. Apparently, the lignin-derived porous activated carbon showed the well-developed pore distribution with macroporous cores, mesoporous and microporous channels as well as the highest BET SSA of 3775 m2 g−1 with a KOH/carbon ratio of 4:1 at 800°C, as shown in Fig. 4c,d. The as-prepared electrode achieved as high as 286.7 F g−1 at a current density of 0.2 A g−1 in 6 M KOH electrolyte for supercapacitor applications. In another work, Wu et al. developed one-step process to fabricate lignin-based activated carbons, utilizing various activating agents (ZnCl2, KOH, K2CO3) for use in electric double layer capacitors (EDLCs) [68]. The as-treated activated carbons with ZnCl2, KOH, and K2CO3 were calculated to be 142, 251, and 263 F g−1, respectively. In contrast, the electrode without chemical activation showed a much lower specific capacitance of 69.8 F g−1.
The template method is one of the most common utilized activation techniques to obtain porous activated carbon materials with well-defined and cross-linked porous structures as well as tailored pore size distribution [23,58]. It is noted that the original carbon precursor with a small surface and a relatively low capacitance can be increased after template methods [58]. For example, Song et al. synthesized lignin-derived mesoporous carbon using a nano-MgO template with Pluronic F127 [70]. Interestingly, they utilized vacuum drying and freeze-drying in order to ensure sufficient disperse of Mg template during solvent evaporation. The as-prepared electrode displayed the BET SSA of 712 m2 g−1 and total pore volume of 0.9 cm3 g−1, respectively. The specific capacitance of the electrode reached 186.3 F g−1. In another work, Ma et al. investigated an interesting strategy, in which reduced materials size can be an effective to enhance the electrochemical performance [71], as illustrated in Fig. 4e. They prepared lignin-based ultrafine porous carbon nanofibers (LUPCFs) through an electrospinning process, utilizing a combination of lignin and polyvinylpyrrolidone (PVP) as the carbon source and incorporating zinc nitate hexahydrate (ZNH) as an additive. ZNH serves as template to generate micropores with the increase of SSA and to provide positive effects on the graphitization degree and surface N and/or O contents. As shown in Fig. 4f, the quantity of adsorption at low relative pressure was gradually rise with the introduction and subsequent increase of ZNH, indicating that ZNH was transformed into ZnO nanoparticles. This was supporting the fact that ZNH play an important role in producing micropores with the increase of SSA. The asobtained LUPCFs were calculated to be 217 F g−1 at 0.1 A g−1 and 152 F g−1 at 20 A g−1, respectively. Interestingly, the LUPCFs electrode delivered excellent capacity retention of 92% after 10000 cycles (Fig. 4g).
4.3. Composites with metal oxides and conducting polymers
In recent years, metal oxides and conducting polymers gradually attracted attention due to their high specific capacitance. However, metal oxides and conducting polymers faces several limitations in terms of their suitability to large-scale systems due to factors such as high cost, insufficient cycling performance, and inconsistence in process [95,96]. Consequently, composite materials comprising metal oxide and/or conducting polymers with carbon-based materials have been explored as potential electrode materials that offer high capacitances and enhanced stability, achieved through the synergetic combination of the impressive conducting and mechanical properties possessed by their individual components [97,98]. For example, Chen et al. introduced a liquid-crystal-line (LC) phase-templating method to fabricate self-assembled NiO nanoparticles in lignin-derived mesoporous carbons using formaldehyde and glutaraldehyde as cross-linking agents [99]. The NiO@mesoporous carbon (MPC) composite materials showed metal oxide contents in the range of 49–79 wt% and high SSA of 503–802 m2 g−1. Moreover, NiO@MPC composites displayed uniform pore sizes (≈3.7 nm), various pore distributions and large pore volumes (0.46–0.68 cm3 g−1). The results demonstrate that the NiO@MPC composites reached high specific capacitance of 880.2 F g−1 at a 1.0 A g−1 and displayed good retention of 93.7% after 1000 cycles. In another work, Ma et al. prepared MnO2-decorated lignin-derived carbon nanofiber mats (ECNF/MnO2) as the binder-free supercapacitor electrodes [100]. Fig. 5a–c shows the designable morphological structures with ECNF/MnO2 ratio of 1:1. The freestanding and highly graphitic electrospun carbon nanofiber (ECNF) revealed a fiber diameter of ~200 nm and BET SSA of ~583 m2 g−1. The as-prepared ECNF/MnO2 mats/electrodes showed an impressive gravimetric specific capacitance of 83.3 F g−1 at a current density of 0.25 A g−1. The symmetrical supercapacitor, assembled using the ECNF/MnO2 mats/electrodes, showed an energy density of 84.3 Wh kg−1 along with a power density of 5.72 kW kg−1. In another interesting work, Yu et al. investigated the utilization of iron oxide particle-decorated on hollow carbon nanofibers (HCNFs) derived from lignin. This was achieved through a coaxial electrospinning method, where a core of poly (styrene-co-acrylonitrile) solution was enveloped by a shell made of acetic acid lignin [101]. In their work, iron (III) acetylacetonate serves as the iron oxide-precursor additive in the shell. The prepared electrode displayed a high specific capacitance of 121.5 F g−1 at a current density of 0.5 A g−1 and excellent cycle stability with capacitance retention of 90% after 1000 cycles. The good electrochemical performance of HCNFs was mainly attributed to the interactions between iron oxide and electrolyte ions in chemical reactions and the hollow structures, which play a substantial role in providing with more active site and pathways for rapid transportation of electrolyte ions. Recently, Zhou et al. developed an intriguing type of an activated interconnected lignin-derived carbon fibrous network (AILCFN) as electrode materials via electrospinning method followed by thermal treatment [102]. First, they isolated the kraft lignin from the kraft pulping process. Then, the activated interconnected lignin carbon fiber (AILCFN) was prepared by electrospinning and carbonization process. The as-obtained AILCFN-3 demonstrated remarkable performance, with a capacitance of 0.41 F cm−2 when subjected to a current density of 0.2 mA cm−2, equivalently or 278.9 F g−1 at 0.14 A g−1. This sample also exhibited an excellent rate capability, maintaining 69% at the current density from 1 to 20 mA cm−2. The resulting AILCFN/Ni-Co-S composite showed a high specific capacity of 1140.0 C g−1 at a high current density of 10 A g−1. The assembled hybrid device with AILCFN electrode and AILCFN/Ni-Co-S composite electrode for flexible solid-state battery-capacitor exhibited a high energy density of 30.8 Wh kg−1 at power density of 0.8 kW kg−1 with good cycle stability and flexibility.
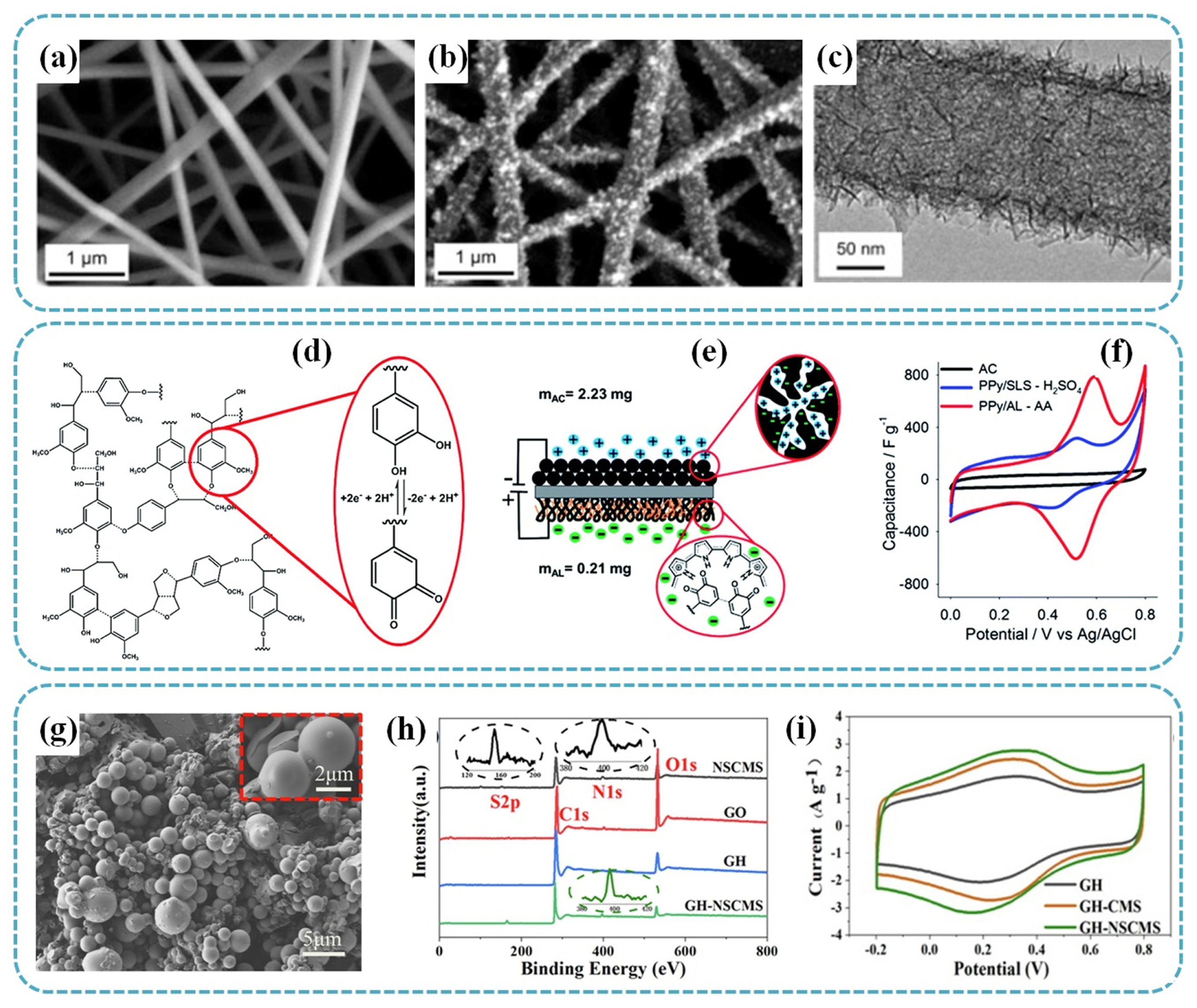
SEM images showing the morphological structures of (a) ECNF and (b) ECNF/MnO2 (1:1). TEM image showing the typical morphological structures of MnO2 nanowhiskers on fiber surface of (c) ECNF/MnO2 (1:1). Adapted from Ref. [100] with permission from Elsevier B.V. (d) Illustrative schematic of lignin structure along with the redox process occurring between hydroquinone/quinine (QH2/Q). Aromatic methoxy groups are converted to hydroxyl groups during the initial voltammetric cycles. (e) Illustrative schematic of PPy/AL-AC cell. (f) Single-electrode CV profiles normalized to specific capacitance (10 mV s−1) of electrode prepared on stainless foil. Adapted from Ref. [106] with permission from Royal Society of Chemistry. (g) SEM image of NSCMS. (h) XPS spectra analysis of NCSCMS, GO, GH, and GH-NSCMS. (i) CV curves of GH, GH-CMS, and GH-NSCMS at 10 mV s−1. Adapted from Ref. [112] with permission from Elsevier B.V.
Due to their unique structures and outstanding electrochemical properties, which include good redox reversibility, high theoretical capacity, and high electron transfer kinetics, quinones and their derivatives have been receiving wide attention as potential electrode materials for renewable energy systems [103]. Therefore, considering that lignin is a phenolic rich redox active polymer that can store charges through a reversible redox reaction [23,103], the redox process involving the quinone structures within lignin and its derivatives offer alternative approach for generating capacitance. Several investigations have demonstrated that when conductive polymer electrodes are combinded with lignin derivatives, they induced the formation of quinone groups. These quinine groups, in turn, contribute to improve the charge storage capacity and capacitance of electrode materials derived from lignin [27,104,105]. Significantly, Milczarek and Inganäs investigated a goup of materials that involve the integration of PPy and LS with redox functionalilites [27]. They have demonstrated that interconnected networks formed by combining LS and PPy are applicable for storing charges and energy storage. It was shown that quinone group in lignin play a role in storing and exchanging electrons and protons during the redox cycling process in a composite of an electroactive conjugated polymer/biopolymer. Inspired by the first work from Milczarek and Inganäs on the use of redox capacity in lignin, Admassie et al. reported the electrochemical and charge storage properties of a ternary system consisting of PPy, a lignin derivative and phosphomolybdic acid (HMA), H3PMo12O40·nH2O in biopolymer electrodes by electrochemical deposition [104]. It is noted that the metallic acids played a role in enhancing stability by minimizing the volume changes when solvents and ions from electrolyte solutions removed. Furthermore, incorporating HMA result in a rise in the specific capacitance of the PPy-lignin composite, increasing it from 477 to 682 F g−1 when evaluated at a discharge current of 1 A g−1. In another work, Leguizamon et al. explored the use of alkali lignin (AL) to increase the capacitance of composite polymer with the redox processes using acetic acid as the deposition solvent [106]. The illustrative schematic structure of lignin along with redox processes occurring between hydroquinone/quinine (QH2/Q) is shown in Fig. 5d. They assembled an asymmetric supercapacitor with PPy-lignin cathodes and AC anodes (Fig. 5e). Apparently, compared to PPy/sodium lignosulfate (SLS) composite, the electrode containing PPy/AL showed an increase in capacitance from 312 F g−1 to 444 F g−1, as shown in Fig. 5f. The enhanced electrochemical performance of the PPy/AL electrode was attributed to the addition of AL with higher phenol content.
Poly(3,4-ethylenedioxythiophene) (PEDOT) has been investigated as a compatible conducting polymer for lignin due to its physicochemical properties, such as high electronic conductivity, electrochemical and thermal stability [107,108]. For another example, Ajjan et al. introduced two synthetic routes, chemically and electrochemically polymerization of PEDOT with lignin [107]. They found that the addition of the hydroquinone/quinine redox couple was important to achieve the enhanced electrochemical performance. The PEDOT doped lignin showed the specific capacitance of 170.4 F g−1, which is doubled to the reference of PEDOT electrode (80.4 F g−1). Moreover, the as-prepared PEDOT/lignin biocomposites exhibited good cycling stability, retaining 83% at current density of 8 A g−1 after 1000 cycles. In another work, Navarro-Suárez et al. fabricated two different all-cell supercapacitors utilizing natural lignin-electrode materials and partially reduced graphite oxide (prGrO) [109]. They used prGrO and lignin/PEDOT as negative and positive electrode in an asymmetric device, respectively. Moreover, a composite material comprising both susbstances was synthesized and examined within a symmetric configuration. The electrochemical performance indicated that the asymmetric device achieved notably high cell capacitance of 34.6 F g−1 at 0.1 A g−1, when compared to symmetric cells composed solely of lignin/PEDOT and properties of the full cell.
The incorporation of lignin with graphene and carbon nanotubes (CNTs) is an effective approach for enhancing the electrochemical performance of lignin-based electrode materials. This is because phenylpropanoid subunit in 3D spatial structure of lignin can play a significant role to inhibit the restacking of the graphene sheets due to its π–π interactions [97]. For example, Cai et al. investigated lignin-based biochar/graphene composites through a facile and rapid coprecipitation method [110]. It was discovered that during the co-precipitation and carbonization processes, lignin might produce a tiny amount of graphitic framework to enhance conductivity and prevent GO from restacking. The carbonized lignin–GO composites have a high specific capacitance as electrode materials. At a scan rate of 20 mV s−1, carbonized lignin–GO was determined to have a specific capacitance of 103 F g−1. In another work, Ye et al. exploited lignin as a reductant and morphology directing agent to fabricate 3D lignin/reduced graphene oxide (RGO) composite via hydrothermal carbonization [111]. The as-prepared 3D lignin/RGO composite with interconnected porous network displayed a high specific capacitance of 133.9 F g−1 at 10 A g−1 with high SSA of 1804 m2 g−1. Note worthily, the 3D lignin/RGO electrode showed excellent capacitance retention of 86.5% after 1000 cycles. More recently, Cui et al. investigated a 3D N, S codoped lignin-based carbon microsphere/graphene composite hydrogel (GH-NSCMS) as electrode materials using corn cob lignin through hydrothermal method [112]. The as-prepared N, S co-doped lignin-based carbon microstructures (NSC) possessed a smooth surface with the uniformly distributed small-sized particles (2–5 μm), as displayed in Fig. 5g. XPS analysis showed that nitrogen and sulfur were successfully loaded onto carbon spheres in Fig. 5h. With the combination of N, S co-doped carbon microsphere and RGO with 3D “sphere-in-layer” morphology, the as-obtained GH-NSCMS electrode displayed good electrochemical performance (Fig. 5i), exhibiting superior specific capacitance of 434.6 F g−1 at 0.5 A g−1 with excellent capacitance retention of 94.15% after 5000 cycles.
5. Summary and Outlooks
In summary, lignin-derived porous carbon materials are promising and functional electrode materials for SCs with tailored morphologies, structures, high carbon contents, convertibility to aromatic compounds, low cost, and environmentally friendly nature of lignin and its derivatives, the lignin-derived porous carbon materials are promising electrode materials for SCs. Moreover, recent studies have demonstrated quinone functional groups in the lignin are mainly responsible for the redox activity of electrode materials via electron exchange. Thus, it is not unexpected that the development of lignin-derived porous carbon electrode materials with excellent electrochemical performance in SC applications has soared over the past years. Nevertheless, there are still some drawbacks of lignin-derived porous carbon materials. For example, the variety of lignin form from different sources and by separation method hinders their practical applications, resulting in a random distribution of lignin chains and unsatisfactory physicochemical properties of raw lignin, such as high molecular weight and low purity. It is well known that the physicochemical properties of lignin and its derivates from the separation process of raw lignin precursor play a substantial role in stable and superior performance of the lignin-derived porous carbon electrode materials for SC applications. Therefore, more efforts are needed in this field to improve the electrochemical performance of lignin-derived porous carbon materials and their derivatives for practical application in future SCs. Several current challenges and prospective solutions are described as follows.
The precise design and synthesis of lignin-derived porous carbon materials with uniformly controlled in pore size distribution and porosity, including from the pure and pristine materials to their elaborate composites, should be addressed to realize advanced electrode materials with superior electrochemical properties for efficient SC applications.
Although numerous lignin-derived porous carbon materials have been developed, there is still a lack of their diversity in compositions and structures. Therefore, optimizing existing synthetic methods and exploring new synthetic strategies to enrich the structures and composites are essential research directions. Especially, modifying the physiochemical properties of lignin precursor materials by controlling separation and/or activation process would enable the functional design of lignin-derived porous carbon materials for improvement of electrochemical performance in SCs. Moreover, combining metal/conducting carbon components with lignin-derived carbon to form composites could effectively overcome the drawbacks of individual components to realize multiple functionalities. Incorporation of functional carbon materials, such as conducting polymer, CNTs, and graphenes, could enhance the electrochemical performance of lignin-derived porous carbon materials. These functional materials could introduce synergistic effects into the lignin-derived porous carbon materials to provide opportunities to achieve more potential properties in a single material, which could maximize the potential of lignin-derived porous carbon materials for SCs.
The application of advanced technologies, such as STEM, X-ray absorption spectroscopy, In- and Ex-situ Raman, In-situ electrochemical impedance spectroscopy (EIS) and NMR spectroscopy for the analysis on the composition, structure, and physico-chemical properties of lignin-derived porous carbon materials, is also imperative to gain a deeper understanding of the mechanism of their functions. In turn, the discovery of the structure-performance relationship would guide the optimization of processing strategies of lignin-derived porous carbon materials for enhanced electrochemical performance.
Despite these challenges, the accomplishments covered in this mini review are indeed encouraging. With persistent research contributions and technological innovation, we should witness the development of new ESS technologies as well as further applications of lignin-derived porous carbon materials in the fields of renewable energy and environmental science in the near future.
6. Conclusions
In conclusion, owing to controllable morphologies, structures, high carbon contents, convertibility to aromatic compounds, low cost, and environmentally friendly nature of lignin and its derivatives, the lignin-derived porous carbon materials are promising functional materials for SCs. Thus, it is not surprising that the development of lignin-derived porous carbon materials with excellent electrochemical performance in SC applications has exploded over the past years. Also, this review concludes with some of our own perspectives on the present major challenges and their potential solutions, hoping to galvanize continuous innovations and valorizations for the future development of lignin-derived porous carbon electrode materials for SCs.
Acknowledgment
This work was supported by the Industrial Strategic Technology Development Program (20012763, Development of petroleum residue based porous adsorbent for industrial wastewater treatment) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea). This work was also supported by the Technology Innovation Program (RS-2022-00156080, Development of electrical double layer capacitors for power supplement of hydrogen forklift) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea).
Notes
CRediT authorship contribution statement
Hae Woong Park: Conceptualization, Writing - original draft. Hyo-Jun Ahn: Writing - review & editing. Kwang Chul Roh: Supervision, Writing - review & editing.