Revolutionizing Energy Storage: Exploring Processing Approaches and Electrochemical Performance of Metal-Organic Frameworks (MOFs) and Their Hybrids
Article information
Abstract
The text highlights the growing need for eco-friendly energy storage and the potential of metal-organic frameworks (MOFs) to address this demand. Despite their promise, challenges in MOF-based energy storage include stability, reproducible synthesis, cost-effectiveness, and scalability. Recent progress in supercapacitor materials, particularly over the last decade, has aimed to overcome these challenges. The review focuses on the morphological characteristics and synthesis methods of MOFs used in supercapacitors to achieve improved electrochemical performance. Various types of MOFs, including monometallic, binary, and tri-metallic compositions, as well as derivatives like hybrid nanostructures, sulfides, phosphides, and carbon composites, are explored for their energy storage potential. The review emphasizes the quest for superior electrochemical performance and stability with MOF-based materials. By analyzing recent research, the review underscores the potential of MOF-based supercapacitors to meet the increasing demands for high power and energy density solutions in the field of energy storage.
1. Introduction
In recent years, global energy demand has risen due to higher consumption in population, industry, and households. To meet this demand, new methods are being explored. Fossil fuel combustion, like coal and natural gas, has historically been a major energy source. A sharp decline in natural reserves has been perceived during recent years [1–6]. Keeping in view the current situation of exploiting natural resources in an illimitable manner, new ways of meeting the energy demands provoked the curiosity of researchers worldwide.
One of the alternatives to deal with the energy demands is using Renew. Energy resources such as wind, solar, tidal and biomass, etc. [7–13]. Pros and cons of consuming Renew. Energy resources involve their renewable nature while the availability of limited amounts of energy along with their intermittent nature is a serious disadvantage [14–17]. As the world is shifting towards advancements such as the development of hybrid electric vehicles [18–22] and the use of other smart technologies, an exceptionally colossal amount of energy is required. Advanced technologies require the provision of high energy and power density for their continual operation. Unfortunately, this need can’t be accomplished by using renewable energy resources alone.
Initially, supercapacitors (SC) had attracted researchers to provide appreciable energy and power density involving the conversion of chemical to electrical energy while helping to store these charges [23,24]. Lamentably, supercapacitors in their primary form were unable to cover high energy and power aspects alone, simultaneously [25–27]. Supercapacitors provide high energy density occasionally but low power density and vice versa [28,29]. Researchers felt a need to modify and develop new materials to be used so that the requirement of high energy and power density can be met concomitantly using supercapacitor materials.
Metal-organic frameworks (MOFs) have provided a breakthrough over the past few decades to meet both energy and power density needs simultaneously. MOFs are a class of materials that have been known for their exceptional energy and power densities [30–32]. Their extraordinary properties involve their porous nature [33–36], high thermal and electrical conductivities [37–39], tunable nature, and large specific surface area [40,41]. They are mainly comprised of metal atoms that are linked by using organic linkers. They have been used widely for gas storage [42,43], energy storage [44–46], water splitting [47], medical purposes [48,49], soil pollutants removal [50,51], water purification [52,53] and biosensors [54,55]. MOFs can be classified into monometallic, bimetallic and trimetallic on the basis of availability of number of metal precursors. Literature showed that presence of more oxidation states helps to improve the performance of MOF as it contributes in electron transfer. Based on this general observation the superior properties of trimetallic MOFs make them potential candidates for the energy storage process [56]. On the other hand, carbon based MOFs generally present the pseudocapacitive behavior thus enhancing the efficiency of MOFs [57]. While MOF derived sulfides and phosphides also provide excellent electrochemical properties, tunable pores with large available surface areas having an advantage of better catalytic activity [58].
In this review, we provided a broader overview of MOFs and their derivatives with recent developments in the field based on morphological characteristics and synthesis approaches. Several early reviews reported their implementation in different fields regarding electrochemical energy [59,60] but their most important uses are in supercapacitors (SCs) for accomplishing the target of high energy availability. We hope that this review provides the basis for further developments in MOFs keeping in view the challenges thus improving their overall performance parameters in a better way.
2. Experimental
Due to their distinctive structural and functional characteristics, MOFs have gained significant recognition as a substantial class of porous compounds. These structures are formed by linking organic linkers and metal ion clusters, and the choice of primary building blocks (PBBs) plays a vital role in determining the MOF’s ultimate properties.
2.1 Chemical vapor deposition (CVD)
The CVD process assists in the synthesis of hybrid MOFs with components in vapour form producing high-porosity materials with uniform structural morphological outcomes [61]. Owing to extraordinary properties such as large crystal structures with one-layer thickness graphene was manipulated using CVD for exfoliation of graphite structure, reducing rGO and graphite oxidation that helped in the charge transfer process [62]. Combining graphene with multi-walled CNTs manipulating CVD, supercapacitors were fabricated showing excellent electrochemical properties by Kalam et al. [63]. Hybrid structures of CNTs and graphene were also produced using this process where magnesium oxide was used as a catalyst thus providing conductive paths for electron charge transfer [64]. Preparation and growth of single crystal MOF of Mo2(INA)4 has been reported by Claire et al. [65] with excellent performance. Cao et al. [66] also used the CVD method for the preparation of ZIF-67 MOF and fabricated an ASC device of Ni@Z67. Legenstein et al. reported Cu-INA [67] synthesis using CVD while 3D graphene MOFs [68] and Ni@ MOF [69] have also been prepared using this method.
2.2 Hydrothermal/Solvothermal method
This environmentally friendly process is best utilized for close system diffusivity. For the preparation of designer precipitates such as high-purity particles, and particles with controlled chemical and physical characteristics, this process is widely used. It requires less amount of energy with low temperatures along with the advantage of easy implementation with scaling [70]. MOFs involving Ni-MOF [89], Co & Cd MOFs [71–75], Ni-Co MOF [76], Ni-Co-Mn MOF [119], Ni-Mn@C [107], Zr-Ce-Ni MOF [109], Ni-MOF with CNTs [111], Mn-MOF with CNTs [112], etc. have been reported by the researchers prepared using this facile method.
2.3 Microwave-assisted method
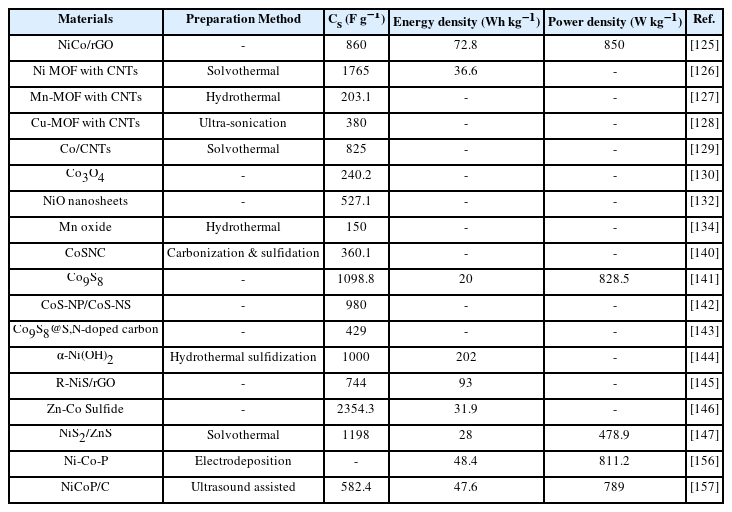
Microwave-assisted processes can be used to create small metal and oxide particles. The solution is exposed to microwaves and allows precise control over the size and shape of MOFs being synthesized [77,78]. Synthesis of ZIF-8 MOF has been reported by Xu et al. [79] thus obtaining significant outcomes by noting a reaction time reduction from 48 hrs. upto 10 minutes. A report from Wang et al. [80] also described the synthesis of MIL-100 (chromium). The valuable findings revealed a time reduction in comparison to the solvothermal approach with improved yield. Weiyang et al. [81] also made use of this method to prepare SnO@Zr hybrid MOF structures with a reported capacity of 994 mAh g−1 for energy storage applications in batteries. Ni-MOF [88] with a reported energy density of 33 Wh kg−1 has also been manipulated by using this method. The fastest MOF synthesis of MIL-53 [82] is also one of the beneficial results of this technique. Two studies reported MIL-101 chromium synthesis also using this method [83,84].
2.4 Ultrasound-assisted method
It is a powerful technique for the preparation of MOFs, which involves the use of high-frequency ultrasound to promote the synthesis of MOFs. In this method, the metal precursors are mixed and subjected to ultrasonic waves that assist in nucleation and crystal growth under cavitation and temperature effects. Various MOFs have been prepared for energy storage applications such as supercapacitors that involve Cu-MOF [85] with H6TDPAT as a linker in DMF solvent. Along with this other MOFs such as Co-MOF [86], Mn-MOF [87] and Ni-MOF have also been prepared using an ultrasound-assisted method.
2.5 Precipitation method
For the preparation of huge amounts of powder samples, this method can be very useful as it provides basis for the formation of precipitates which are formed due to an excess quantity of solute particles in a solvent breaching the solubility limit. The fast rate of precipitation due to high temperature puts limitations against the morphology regulation of particles to be prepared. Using this facile technique preparation of magnetic nanoparticles of cobalt-iron oxide [88], nickel phosphate deposited on GO [89] has been reported exhibiting (approx.) 1330 F g−1 at a small current density of 0.5 A g−1. Zn-MOF was prepared by Liu et al. [90] using this method reporting an energy density of 22.4 Wh kg−1. Also, MOF-5 [91], CeO2 from Ce-MOF [92] and ZIF-8 MOF in Na-ion batteries are few reported researches in a recent era using the precipitation method.
2.6 Slow diffusion process
For achieving highly crystalline MOFs slow diffusion process is often employed. The steady and gradual mixing of precursors is achieved in this process with elevated electrochemical performance often employed due to slow diffusion species in MOF preparation process. Better electrochemical performance of MOF can be attributed to efficient diffusion of precursor ions. Preparation of Cu based MOFs have been reported by Rajak et al. using amalgamation of ligand approach while MOF derived cobalt iron oxide with MXene have also been reported in the literature by Xie et al. recently [93,94]. Moreover, using concurrent approach of using mix ligand, polymer reinforced Zn based MOF have been reported by manipulating slow diffusion process [95].
2.7 Sulfidation and carbonization process
Reaction of Sulphur containing compounds or the introduction of Sulphur in MOF structures can be used to enhance the electrochemical properties of MOF derived materials. Sulphur being advantageous for having more energy density and highly active oxidation/reduction material can be of more value. While enhancing the stability and conductivity of MOF derived structures a heat treatment process of MOF sulfides brings efficient results to the whole scenario of energy storage. Treatment of ZIF-67 with sulfur caused the conversion of Co-MOF into CoS through sulfidation reactions [96] while the reaction of titanium oxide with ZIF-67 MOF produced CoS structures with hierarchical morphology [97]. Production of porous carbon based nanostructures have been achieved by carbonization treatment of iron carbide nanocrystals with a large surface area for efficient charge storage capability [98].
3. Metal-organic framework (MOFs) for supercapacitors
The general storage mechanism involved in SCs based on MOF based materials involve two mechanisms: Electrical double-layer charge storage (EDLC) and pseudo-capacitive mechanisms. EDLC stores electrical charges using electrostatic forces on the internal surfaces. Carbon-based materials such as graphene [99,100], CNTs (carbon nanotube) [101] and mesoporous carbon [102,103] show EDLC behavior. Reversible fast surface redox reaction charge storage mechanisms have been shown by pseudo-capacitors. Transition metals and polymers with conductive nature show pseudo-capacitive behavior. High conductivity and provision of large surface area for ions can be achieved by manipulating the tunable nature of MOFs thus proving them as a promising electrode material for electrochemical applications.
3.1 Monometallic MOFs for supercapacitors
Monometallic MOFs with satisfactory surface areas and small pore sizes can be beneficial for energy storage applications. Mofokeng et al. synthesized Ni-MOF using a microwave-assisted technique reporting an energy density (33 Wh kg−1), power density (983 W kg−1) and a specific capacity of 199 mAh g−1. Carbonizing along with chemical etching was carried out on prepared Ni-MOF for a reduction in nanoparticle size [104]. Excellent cycling stability with only a 12% loss was observed in a 2-electrode assembly with less series resistance. The better performance of Ni-MOF was attributed to flake-like morphology revealed by SEM along with the induction of oxygen vacancies. Li et al. fabricated Ni-MOF by solvothermal approach with BTC (1,3,5-benzene tricarboxylic acid) as a ligand. They reported an excellent specific capacitance of 977.04 F g−1 with only an 8% cycling stability loss. Exposure of (001) crystallographic plane provides the shortest ions transportation path that contributed to the remarkable performance of electrode material [105]. Lee et al. [106] synthesized Co-MOF with pseudocapacitive nature that revealed an energy density (7.18 Wh kg−1) with a specific capacitance value of 206.76 F g−1. SEM images showed a porous uniformly distributed structure that provided easy pathways for ions movement from which the relationship between molecular linker length and pore size along with the surface area was explored by the same group [107]. Their findings produced that longer-length molecular morphologies provide a large surface area for ions to settle thus providing better electrochemical properties. Benzene dicarboxylic acid (BDC), 2,6-naphthalene dicarboxylic acid (NDC) and 4,4-biphenyl dicarboxylic acid (BPDC) with varying lengths were used as organic linkers to link cobalt atoms. Indium-based MOF was synthesized by Du et al. [108] using indium nitrate (In (NO3)3) with a reduced pore size of 3 nm along with excellent stability. A specific capacitance of 150.2 F g−1 was reported at 0.2 A g−1 against 437-MOF which was credited to a large surface area supported by an ordered porous structure.
3.2 Binary MOFs for supercapacitors
Considering the inferior performance of monometallic MOFs, Pan et al. [109] reported the doping of manganese (Mn) in Ni for making an attempt to reduce the conduction path between Ni and Mn ions using a binary metal approach. 1.89 mAh cm−2 was reported by Pan and his co-workers at 1 mA cm−2. Two-electrode assembly of Ni-Mn-MOF//EDLC (AC) yielded an energy density of 67.5 Wh kg−1 along with a remarkable 84.5 W kg−1 power density bearing 6% capacity retention loss. Diaz et al. [110] synthesized Co8-MOF-5 with varying amounts of MOF that exhibited pseudo-capacitive behavior generally shown by EDLC materials.
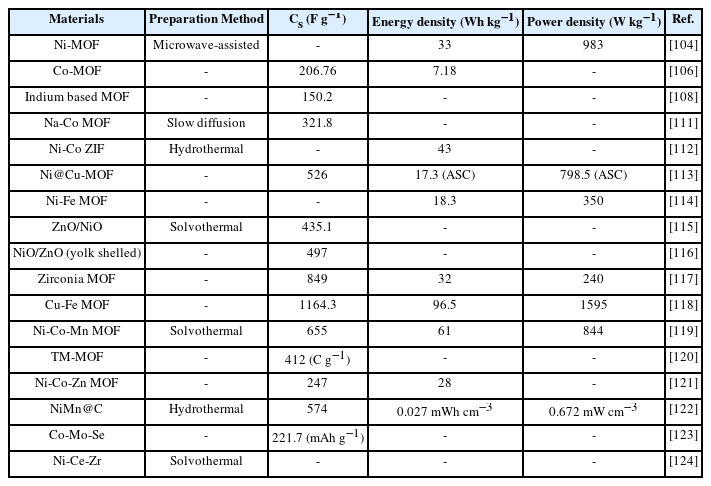
Rajak et al. [111] took a step to synthesize heterometallic Na-Co-MOF using a facile slow diffusion process with an approach of using mixed ligands namely, Azopy (4,4′-Azopyridine) and SDCA (2,5′-thiophene carboxylic acid). The specific capacitance obtained after electrochemical analysis was 321.8 F g−1 at a current density of 4 A g−1 with 21% capacitance loss. The better performance of MOF was attributed to the block-like morphological features. Hussain et al. fabricated Ni-Co ZIF (Fig. 1(a)) with CuO using the dry oxidation method achieving 43 Wh kg−1 of energy density. Decoration on CuO surface with forest-like Ni-Co ZIF provided accessibility to pores providing highly active redox sites showing incredible electrochemical performance [112].
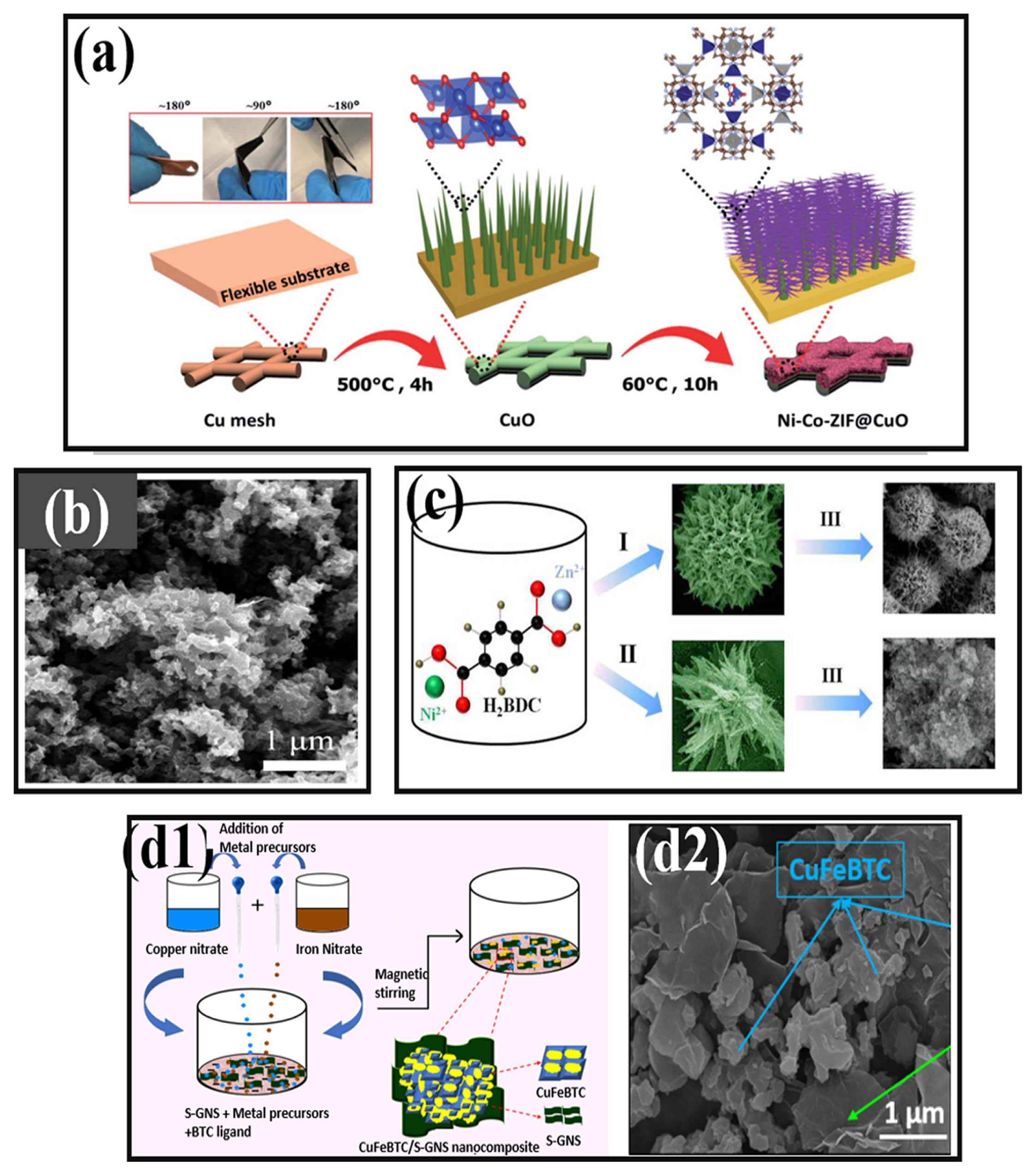
(a) Synthesis of Ni-Co ZIF@CuO [112]. (b) SEM images of Ni-Fe bimetallic MOF [114]. (c) Schematic illustration of the fabrication process for three dimensional NiO@Au@ZnO hybrid nanostructure and its corresponding SEM image [115]. (d1,d2) Schematic of Cu-Fe BTC MOF preparation and its corresponding SEM image [118]. [Images reprinted with permissions]
A comparison was made between the performance parameters of Cu-MOF and Ni@Cu MOF by Wang et al. [113]. Ni@Cu-MOF showed a very smaller pore size in contrast to Cu-MOF found by BET which formed the basis of marvelous performance. A way much higher specific capacitance value of 526 F g−1 was calculated than pristine Cu-MOF (126 F g−1). The energy density shown by the asymmetric supercapacitor (ASC) device was 17.3 Wh kg−1 at an incredible power density of 798.5 W kg−1. Fu et al. [114] successfully synthesized a Ni-Fe MOF using precursors of iron chloride (FeCl3) and nickel chloride (NiCl2) by carbonizing polymer linkers. Better morphological features with large surface area contributed to charge transportation (1049.51 m2 g−1) revealing an energy density of 18.3 Wh kg−1 along with a maximum power density of 350 W kg−1. The synergistic effect of Ni and Fe ions showed coral cross-linked structure with small pore size observed from SEM image (Fig. 1(b)). Making use of the solvothermal process followed by calcination with precursors of Ni-Zn MOF, the formation of seaweed-like morphologies of ZnO/NiO was attempted [115]. Investigation have been carried out on changing the molar ratios with an effect on characteristic morphology of specified MOF. SEM images (Fig. 1(c)) revealed the seaweed-like structure that assisted in ion transport efficiently thus exhibiting a specific capacitance of 435.1 F g−1 at 1 A g−1.
Li et al. [116] used a solvothermal method to prepare a yolk-shell hollow structure comprising of bimetallic MOFs, NiO/ZnO by manipulating a template-based strategy using H2BDC as a linker. 497 F g−1 of capacitance value was reported by their team at 1.3 A g−1 with an excellent stability. SEM analysis showed the formation of uniform spherical structures having a core inside with a shell covering while BET analysis was an indicative of mesoporous structure. The better electrochemical performance was attributed to the hollow volume structure that provides ease in accommodating more ions inside it. Highly porous-natured zirconia (Zr) MOF was synthesized by Gao et al. [117] from Zr-Zn bimetallic precursor. Zinc chloride and zirconia nitrate were used as precursors in the presence of BDC linker which were subsequently soaked in acid to remove Zn-MOF from synthesized Zr-Zn MOF. SEM images showed a lot of porous sites with sponge-like morphology assisting in ion transport. Ultimately, better electrochemical performance was reported by researchers including 849 F g−1 of capacitance with energy density (32 Wh kg−1) and power density (240 W kg−1). Rajpurohit et al. [118] investigated Cu-Fe MOF nanocomposite with sulphur-doped graphene using BTC as a ligand aiming to achieve a super capacitive behavior of the synthesized material. Rod cube-like structures (Cu-MOF) with some agglomerated particles (Fe-MOF) showing a vast number of porous sites in SEM analysis was revealed. Electrochemical results exhibited 1164.3 F g−1 of specific capacitance with an extra ordinary 96.5 Wh kg−1 and 1595 W kg−1. Such remarkable electrochemical performance was attributed to the porous structures of nanocomposite with high electrical conductivity of sulphur doped graphene as shown in Fig. 1(d1,d2).
3.3 Tri-metallic MOFs for supercapacitors
Keeping in view the compromised performance of mono as well as bi-metallic MOFs, Han et al. [119] put an effort to upgrade the electrochemical performance of MOFs by working on ternary MOFs. Ni-Co-Mn-MOF was synthesized (Fig. 2(a)) via a solvothermal technique using BTC as an organic linker. The synthesized MOF exhibited a typical battery-type supercapacitive behavior producing 655 F g−1 specific capacitance at 1 A g−1. Due to the excellent layered structure of the as prepared MOF, there was reported approximately 8% capacity retention loss after 10,000 cycles. The ASC device produced a remarkable energy density of 61 Wh kg−1 at 844 W kg−1 due to the availability of more active redox sites confirmed from SEM analysis as shown in Fig. 2(b). Aghazadeh et al. [120] synthesized TM-MOF via cathodic electrodeposition methodology using lanthanum, gadolinium and thulium as metallic precursors. A specific capacity of 412 C g−1 was reported at 1 A g−1 low current density achieving a rate capability of 52.9%. The smooth surface of Ni-foam was observed covered with TM-MOF providing a rough surface morphology showing a thin film growth over it using SEM image. A simple scalable template-based approach was used to prepare ternary MOFs comprising of nickel-cobalt-zinc. The prepared material was subjected to various analysis techniques including SEM, EDX, TEM and XRD. Interconnected porous shell morphology played a crucial role in its charge storage capability. Moreover, in terms of electrochemical performance, a specific capacitance of 247 F g−1 was reported in a coin-shaped assembly. 99% coulombic efficiency retention with an energy density of approximately 28 Wh kg−1 was also reported by Ezeigwe et al. in this studies [121].

(a,b) Schematic of Ni-Co-Mn MOF preparation process and its SEM micrograph [119]. (c,d) Schematic illustration of the preparation of the hierarchical Ni(OH)2–MnO2/C composite and its corresponding SEM image [122]. (e,f) Schematic illustration for the preparation of hollow Co–Mo–Se nanosheet arrays with corresponding SEM micrograph [123]. [Images reprinted with permissions]
A revolutionary tri-metallic MOF NiMn@C was fabricated (Fig. 2(c)) using the combined hydrothermal and carbonization methods [122]. Electrochemical measurements revealed 574 F g−1 of capacitance values showing an elevated energy density (0.027 mWh cm−3) and power density (0.672 mW cm−3). A capacitance retention loss of 13% was reported after subjecting the material to 10,000 cycles. Characteristic nanosheet structures (Fig. 2(d)) with a combined effect of Ni and Mn decorated carbon proved the basis for good electrochemical results. Nanosheets of Mo-Se-Co were prepared on active carbon and used as a MOF precursor material. Excellent supercapacitive properties have been exhibited by ASC device with a specific capacity of 221.7 mAh g−1, and excellent stability with only 5% loss in rate capability after 8000 cycles. Its real application has been tested when it ran a watch for 70 minutes thus showing the capability of prepared electrode material [123]. The synthesis and SEM micrographs can be seen in Fig. 2(e,f). Ni-Ce-Zr trimetallic MOF was synthesized by Meshram et al. [124] using a facile solvothermal technique illustrating an excellent thermal stability of trimetallic MOF when compared to mono and bimetallic MOF combinations.
4. MOF composites with carbonaceous materials
Carbonaceous materials provided a breakthrough in the field of energy storage by providing highly conductive pathways for ion and electron transport.
Graphene-based nanocomposites are focused excensively because of their better electrochemical properties. Several reports have been found for using graphene in amalgamation with MOFs. A nanocomposite of Ni-Co-MOF with reduced graphene (NiCo/rGO) [125] had been produced thus reporting 860 F g−1 of specific capacitance at a current density of 1 A g−1. An energy density of 72.8 Wh kg−1 was obtained at a power density of 850 W kg−1 from ASC device. SEM and TEM showed (Fig. 3(a,b)) the presence of Ni-Co-MOF uniformly well dispersed between gaps of graphene sheets that enhanced the conducting performance of electrode material. This nanocomposite was prepared by using a sonication method to ensure proper dispersion of graphene sheets and to avoid agglomeration of particles.
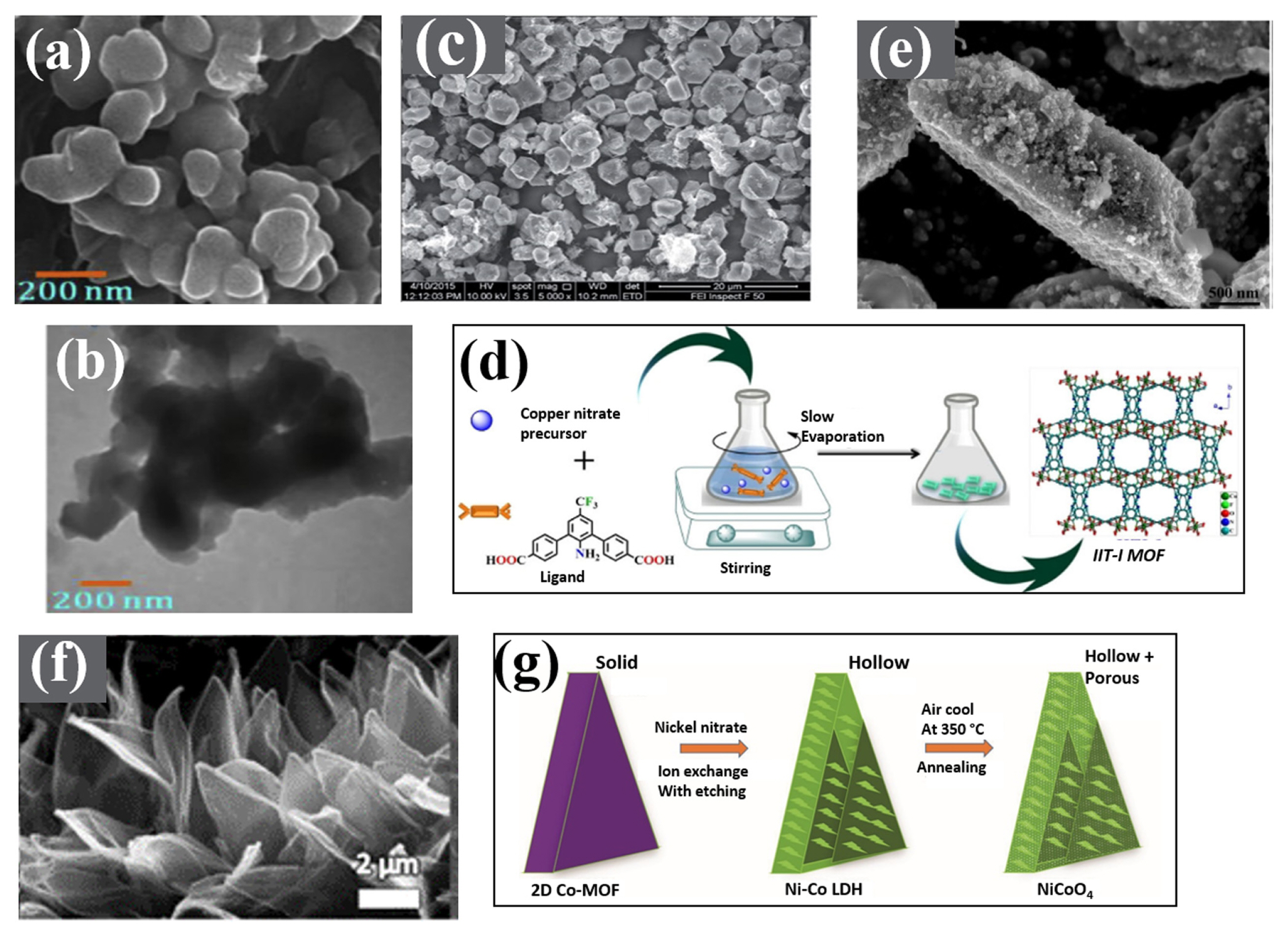
(a,b) SEM and TEM micrographs of Co-MOF [125]. (c) SEM micrograph of Mn-MOF with CNTs [127]. (d) Synthesis of Cu-MOF with CNTs [128]. (e) SEM images of CoS2@ CNTs [129]. (f,g) SEM image NiCo2O4 nanostructure from 2D-Co-MOF solid nano walls with corresponding schematic illustration [136]. [Images reprinted with permissions]
Further, an attempt was made for the fabrication of Ni-MOF with CNTs (carbon nanotubes) by Wen et al. [126]. CNTs provided a long-range conducting path for electron transport in 2 as well as 3-electrode assembly revealing a specific capacitance of 1765 F g−1 along with 36.6 Wh kg−1 of energy density. The widely used hydrothermal method was utilized by Lin et al. [127] for manipulating the incredible properties of Mn-MOF with CNTs. The incorporation of CNTs in Mn resulted in an enhanced electrochemical performance showing a sharp inclination in specific capacitances from 43.2 to 203.1 F g−1. The tremendous performance was attributed to the presence of CNTs, also confirmed by SEM micrographs showing long stick-like structure on blocks of Mn-MOF (Fig. 3(c)). Synthesis of Cu-MOF with CNTs having a specific capacitance of 380 F g−1 was attempted using a newly designed linker 5-(1-oxoisoindolin-2-yl) isophthalic acid (H2L) from Cu(NO3) and CNTs followed by an ultra-sonication technique (Fig. 3(d)) [128]. Expected high specific capacitance was attributed to CNTs addition whose presence was ensured by TEM images illustrating a wrapped morphology of CNTs around Cu-MOF.
Synthesis of Co-MOF by Zou et al. [129] with 1,4-bis(imidazole-1-yl) benzene (bib), 1,3-benzene dicarboxylic acid, (1,2-benzene dicarboxylic acid and 1,4-benzene dicarboxylic acid was carried out to produce CoS2/CNT. A specific capacitance of 825 F g−1 was achieved from GCD at 0.5 A g−1 using CoS2/CNT showing an appreciable pore size and hierarchical morphological features. Maximum dispersion of CoS2 in the carbon pattern shown by SEM served to be the basis for enhanced supercapacitive performance (Fig. 3(e)).
5. MOF-derived metal oxides
Generally, for the preparation of metal oxides, metal cation precursors are dispersed with a 40% by weight ratio in MOFs. Size and shape-controlled metal oxides can be produced by understanding this exceptionally unique feature of MOFs [130]. Synthesis of porous Co3O4 by calcination of MOF crystals [131] by maintaining the morphology of the MOF precursor has been carried out. The capacitance of 240 F g−1 was achieved with only 4% cyclic stability loss from Co3O4 microflowers when used in supercapacitor electrodes. In another study, Li et al. utilized MOF-74 to produce cobalt oxide to be used as an ASC thus producing 647 F g−1 at a current density of 1 A g−1. Further research on utilizing Ni for energy storage was made to obtain superior nickel oxide nanosheets [132] from Ni-MOFs. A specific capacitance of 527.1 F g−1 has been reported at 1 A g−1 with 80% value retention after 5000 GCD cycles. Mn-BTC as a precursor was manipulated by the solvothermal approach [133] to produce Mn2O3 mesoporous nanobars using thin porous sheets. The resulting electrodes exhibited a high specific capacitance of 249.7 F g−1 at a current density of 0.2 A g−1 when used in supercapacitors. Chen and colleagues [134] used pyrolysis to obtain a new MOF called Mn3 (H2O)2(ipa)3 at varying temperatures (500°C and 600°C), resulting in MnOX thus showing 150 F g−1 at 1 A g−1. Chen’s research team [135] was the first to use Zn-Co-MOF to create ZnCo2O4 by calcination for supercapacitor electrodes. A series of NiCoO (oxides) compounds, which were isomorphous to the Co3O4 crystal structure derived from MOF-74 topology phases has been created (MOF-74-Co, MOF-74-Ni, MOF-74-NiCo1, MOF-74-NiCo2, and MOF-74) thus managing to explore the relation between varying metal ratios and electrochemical performance.
Useful work on NiCo2O4 has been reported by Guan et al. [136] following a series of processes that include ion exchange and etching (2D Co-MOF) (Fig. 3(f,g)) obtaining petal like SEM micrograph. Growing porous hollow structures on carbon cloth (CC@ NiCo2O4) delivered 1055.3 F g−1 and at a power density of 2.9 kW kg−1, it showed an energy density of 31.9 Wh kg−1. Valuable contribution in context of energy storage devices has been added by Guo et al. [137] following calcining Co2(BDC)2 MOF for producing (Co0.94Fe0.06)3O4 nanoparticles with hollow nanowires. The capacitance of 169 F g−1 at 1 A g−1 was explored with a low charge transfer resistance shown by as prepared (Co 0.94Fe 0.06)3O4 indicating a value of 0.74 Ω.
6. MOF-derived metal sulfides
MOF-derived metal sulfides as compared to metal oxides provide us with way better stability ratios with enhanced shortened pathways for electron transport due better morphologies [138,139]. Simultaneous carbonization and sulfidation led to the synthesis of cobalt sulfide nitrogen-doped carbon (CoSNC) utilizing porphyrin paddlewheel framework-3 [140]. CoSNC was also observed embedded in carbon matrix in TEM images with 360 F g−1. An effort was put forward by Han et al. [141] to synthesize cobalt sulfide nanosheet array (Fig. 4(a,b)) from ZIF (Zeolitic imidazolate framework) forming a leaf-like morphology. Sulphur produced from hydrogen sulfide (H2S) was made to react with ZIF producing Co9S8 with 1098.8 F g−1 of specific capacitance. Recorded stability was found to be 87.4% over 1000 cycles with the ASC device giving 20 Wh kg−1 at 828.5 W kg−1 of power density. Excellent performance of cobalt urged Hu and his group [142] to manipulate Co-based zeolitic imidazolate (ZIF-67) nanocubes for synthesizing CoS-NP/CoS-NS (Fig. 4(c)). Battery grade material with 980 F g−1 and 88% capacitance retention was obtained. SEM analysis supported by TEM clearly indicated cube-like structures with internal delicate features (Fig. 4(d)). Keeping in view the extensive research on Co-MOF, Liu et al. [143] synthesized Co9S8@S, N-doped carbon introducing nitrogen during pyrolytic treatment of respective MOF. For characterization purposes, SEM, TEM, XRD and XPS were performed to analyze the morphological as well as chemical composition. At a current density of 1 A g−1, the specific capacitance of 429 F g−1 was reported with a good cyclic stability.

(a,b) SEM image of porous Cobalt sulfide nanosheets [141]. (c,d) Synthesis Procedure for Various CoS Hollow Structures with SEM and TEM characterizations [142]. (e,f) Schematic of Ni3S2@Ni preparation with SEM micrographs [144]. (g,h) Schematic illustration of the formation process of the NiS2/ZnS with corresponding SEM micrographs [147]. [Images reprinted with permissions]
Using methyl imidazole for α-Ni(OH)2 as an organic linker, the Ni3S2 followed by hydrothermal sulfidation method was reported by Chen et al. [144] (Fig. 4(e)). A capacitance of 1000 F g−1 with an exceptionally high energy density had been reported. Vertically aligned Ni(OH)2 was seen (Fig. 4(f)) on Ni foam with high flexibility. Since morphology plays an important role in electrochemical performance, following this Qu and his co-workers decorated graphene with NiS NPs [145] using refluxing water MOF (Ni-MOF-74/rGO). A specific capacity of 744 C g−1 was quoted at a current density of 1 A g−1 along with an exceptionally high energy density of 93 Wh kg−1 while retaining 89% capacity retention over a period of 20,000 cycles. The remarkable performance of as-synthesized active material was attributed to its highly porous nature and the presence of reduced graphene oxide (rGO).
To enhance the performance of sulfides a bimetallic approach was introduced to produce Zn-Co sulfide [146] with a leaf-like structures. A comparison between leaf-like structures with and without NS was observed predicting a better performance for uniformly distributed leaf-like structures. The specific capacitance of 2354.3 F g−1 along with only 12% stability loss was reported along with an energy density of 31.9 Wh kg−1. Excellent performance was attributed to conductive paths due to the formation of a nanosheet-like structure. Self-sacrificing Ni/Zn-MOF BDC derived NiS2/ZnS has been fabricated solvothermally followed by subsequent sulfidation at varying time rates to produce varied hollow nanocomposite structures (Fig. 4(g,h)) [147]. Appreciable power (478.9 kW kg−1) and energy densities (28 Wh kg−1) had been reported using BDC as a linker in this research work.
7. MOF-derived metal phosphides
Metal phosphide-based electrodes for supercapacitor applications show excellent electrochemical performance because of their pseudocapacitive behavior, regarding electrical conductivity and remarkable energy densities [148]. Their ease of availability with eco-friendly operation plays a major role in controlling environmental pollution [149–151]. By manipulating UiO-66 MOF, molybdenum nitride, carbide-and phosphides decorated carbon catalysts, can be selectively synthesized without altering the morphology (Fig. 5(a)). This can be achieved through three distinct treatments: nitridation, carbonization, and phosphorization [152]. Zhao and colleagues were able to manipulate the phosphorization method at low temperatures to synthesize iron phosphide-500 (Fig. 5(b)) with 1D-nanorods-like structures using Fe-MOF precursor. These nanostructures are provided with enriched gas-bubbles-releasing sites due to the rich directional channels [153]. A facile though cheap strategy for producing Ni2P and Ni12P5 has been proposed by Tian et al. [154] using Ni-MOF BDC. Ni-Co bimetallic phosphoric hybrids have been synthesized and exhibit excellent performance, due to large porosity with the synergistic effect of Co2P and Ni2P in a bimetallic phosphide system [155].
The excellent electrochemical performance and outstanding stability of the prepared Ni-Co-P nanosheet arrays [156] can be attributed to their structural and synergistic effects. ASC device delivered 48.4 Wh kg−1 along with 811.2 W kg−1 of power density with exceptional cyclic performance (Fig. 5(c,d)). Nanosheets of orderly structured Ni-Co-P/C were fabricated in a tunable manner using Ni-Co-MOF following the phosphorization process. The as-prepared nanosheets produced 583 C g−1 with 37.59 Wh kg−1 at 800 W kg−1. An ASC consisting of NiCoP/C with EDLC has been prepared [157] and it demonstrated an excellent energy density of 47.6 Wh kg−1 at a power density of 798.9 W kg−1, outperforming pristine nickel/cobalt phosphide nanomaterials. A more challenging leaf-shaped heterostructure consisting of cobalt phosphide/cobalt oxide (CoPx)1−y/CoOy was fabricated through the phosphorization of the molecular precursor of 2D ZIF-Co-L [158] using Co3O4 as an intermediate thus revealing 467 F g−1 with 12.7 Wh kg−1 at a power density of 370 W kg−1. Li et al. [159] focused to face the challenge of the availability of more active sites for a better performance of asymmetric supercapacitor (ASC) devices. For this purpose, NiZnCo phosphide derived from NiZnCo layer double hydroxide and ZnCo-MOF have been produced with approx. 11% capacitance loss having an energy density of 62.5 Wh kg−1. Recent work has been reported by Chhetri et al. [160] with an advantage of producing carbon nanofibers as conductive channels in Ni-Fe-P derived from bimetallic NiFe-MOF. The presence of such a characteristic structure with more availability of oxidation states in bimetal ions (Fe and Ni) proved to be the basis of excellent electrochemical activity with 1392 F g−1 having 62.7 Wh kg−1 (energy density) and 8238.2 W kg−1 of power density.
8. Discussion
The electrochemical performance of all the synthesized materials comprising monometallic, bimetallic, and tri-metallic MOFs along with their derivatives have been shown in Table 1 and 2. The major factors found to influence the electrochemical performance of the electrode materials and the devices include nature and type of metallic ions, organic linkers, processing method and conditions, structure, and morphologies, etc. as summarized in Fig. 6. Morphologies such as cubic, shell structure, flower-like morphology and nanosheets like structures were of great concern in providing better electrode performance. A general conclusion regarding MOFs’ performance can be seen from Fig. 7 in which comparisons based on specific capacitance have been sketched. The general comparison showed that the electrochemical performance of MOFs derivatives was of a magnitude way higher than the pristine MOFs. The addition of carbonaceous materials such as GNPs and CNTs provided a breakthrough in improving electron and ion transport aiding in the provision of conductive pathways.

Comparison of electrochemical properties of MOFs and their derivatives on the basis of specific capacitance.
Co-MOF has been reported to show a specific capacitance of 206.76 F g−1 with a uniformly distributed porous structure when compared with bimetallic Zn/Co sulfide (2354.3 F g−1) which showed tremendous performance. The addition of Zn proved to be advantageous regarding the elevation of the electrochemical performance of monometallic Co-MOF. Ni@Co MOF was also proved superior by the researchers when compared with Co-MOF thus providing strong evidence that enhancement of performance can be achieved using hybrid approaches. NiCo/rGO [109] also played its role in improving the electrochemical performance of single metal MOFs showing 72.8 Wh kg−1 synthesized by solvothermal approach. When compared with Ni (33 Wh kg−1) [88] and Co-MOF (7.18 Wh kg−1) [90], a comparatively very high energy density was reported in the literature using carbon incorporated in MOF. Gaps between graphene nanosheets provided better electron movement paths that enhanced the electrochemical activity of MOFs. Similarly, Ni-Co-Mn MOF [103] prepared by the solvothermal method showed 43 Wh kg−1 when compared to Ni-Co ZIF [96] which only gave 43 Wh kg−1 of energy density. Using carbon-based materials generally provide us with good electrical conductivities which was demonstrated when a comparison of Ni-MOF with CNTs [110] and pristine Ni-MOF was made. Ni/CNT showed 1765 F g−1 which is almost double that of Ni-MOF (977 F g−1) synthesized by the facile solvothermal method. Similarly, the effect of adding CNTs is also visible in comparison studies of Co/CNT [113] and pristine Co-MOF [90]. A great difference between specific capacitance was observed because the presence of CNTs provided conductive channels for electron conduction.
Comparison between oxides and MOFs revealed that oxides being more porous and having large surface areas can provide excellent electrochemical properties. Comparing Ni-Zn-Co MOF (247 F g−1) [105] and bimetallic Ni@Cu [97] (526 F g−1) with NiCo2O4 [119] produced 1055.3 F g−1 with 31.9 Wh kg−1 (energy density) which was superior to bimetallic Ni@Co MOF [98] that only gave 526 F g−1 with 17.3 Wh kg−1 when explored by the researchers.
Materials based on sulfides and phosphides provided a breakthrough in this area of energy storage. Their comparison provides tremendous results when compared with pristine MOFs of the same class. In general, we can see from the reported literature that Co9S8 [122] when compared with Co-MOF [90] produced 1098.8 F g−1 which is higher than 206.76 F g−1. Criticizing R-NiS/rGO [126], it also gave proof of better performance than Ni-MOF produced from microwave-assisted method [88] and solvothermal approach [89]. Ni12P5 [135] reported in the literature produced by facile hydrothermal approach proved to be superior in comparison with Ni-MOF in terms of specific capacitance. In this regard, we can check the performance of Ni-Fe phosphate [141] that was produced using the mild-hydrothermal approach with 2086 F g−1 which is also higher than Mn/CNT [111], Ni-Co-Mn [103] and NiMn@C [106].
From the detailed discussion and comparison, the general trend is that the derivatives and composites of energy-storing materials (oxides, carbon-based, sulfides and phosphides) provide better electrochemical performance due to their superior charge storage properties, more conductive paths, and large surface areas in comparison with pristine MOFs. An overview of these comparisons has been presented in Fig. 7. Moreover, the advantages and disadvantages of using various synthesis methods for MOFs have been discussed and listed in Table 3.
9. Conclusions and future work
In summary, a class of MOFs with monometallic, bimetallic, and tri-metallic materials along with their derivatives with a detailed discussion about their electrochemical nature and their morphological characteristics have been taken into consideration. Metal-organic frameworks (MOFs) have diverse applications, including supercapacitors, due to their unique properties like large pore size and surface areas. Tailoring MOFs by altering precursors and processing conditions enhances their electrochemical performance by targeting their morphological features. Hybrid MOFs are pivotal for practical use and boosting materials’ efficiency. Improved electrochemical activity and synergy in MOF derivatives are also emphasized under various synthesis methods and morphological context. The importance of MOFs derivatives has been judged because of their extraordinary structures leading to improved energy and power densities. We hope that this work will serve as a valuable addition to the field of energy storage and pave the way for future developments.
Although very extensive research has been done and is being carried out on MOFs, their derivate and hybrids for energy storage applications, there are still some challenges and aspects that should be focused as listed below:
An improvement in MOF’s energy storage capability during practical phases where harsh conditions (elevated temperatures, changing pressures, etc.) are to be dealt with.
To explore the effect of adding various materials in MOFs on their chemical reactions along with their morphologies.
Cost-effectiveness is also an area of main concern where reduction in cost must not affect the performance of required material.
For preparing hybrid and advanced energy storage materials work should be done on easy and environment-friendly approaches that can be affordable.
Consideration of factors such as MOFs compositions, morphological features, and availability of resources must be considered in an affordable manner.