The Synergistic Effect of 2-Chloromethylbenzimidazole and Potassium Iodide on the Corrosion behavior of Mild Steel in Hydrochloric Acid Solution
Article information
Abstract
The synergistic effect of 2-chloromethylbenzimidazole (2-CBI) and potassium iodide (KI) for mild steel in 1 M hydrochloric acid solution was investigated by potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS). The results showed that, with the addition of 100 ppm potassium iodide, the inhibition efficiecy (IE) of 100 ppm 2-CBI in 1 M hydrochloric acid had been improved from 91.14% to 96.15%. And synergistic parameter of 100 ppm 2-CBI with different amounts of potassium iodide is always greater than 1. The adsorption of potassium iodide combining with 100 ppm 2-CBI obeys to the Langmuir adsorption isotherm. Thermodynamic adsorption parameters, including ΔG0ads, ΔHa and ΔSa of the adsorption of the combinned inhibitor, as well as the Ea of the mild steel corrosion in 1 M HCl with the combinned inhibitor, were calculated.
1. Introduction
Mild steel is widely used in diverse fields of industry, and its corrosion is an unavoidable problem. At present, there are many methods for corrosion protection of mild steel, and corrosion inhibitor is one of the most economical and efficient method [1]. The mechanism of corrosion inhibitors can be roughly divided into two categories. One is to interact with ions on the metal surface to form a precipitated film composed of oxides, hydroxides or insoluble salts and adhere to the metal surface to form a protective film. The chromate and phosphate are typical representative of this type. However, they are often harmful to the human and the environment. Therefore, environmentally friendly “green” corrosion inhibitors are more favorable [2]. Most of the organic corrosion inhibitors have the second mechanism, they slow down the corrosion of the metal by forming an adsorption film on the metal surface [3–5]. And adsorption can be the electrostatic interaction between the charged surface of the metal and the charge of the inhibitor [6], or the interaction between the lone pair of electrons or π electrons in the corrosion inhibitor molecule and the empty d-orbitalson of the metal substrate, namely the coordination bonds [7,8].
2-Chloromethylbenzimidazole (2-CBI) is a benzoheterocyclic compound containing N atom [9], the benzene ring and the N atom bearing the sp2 electron pair are considered as the “anchoring sites” for molecular adsorption at the metal surface [10–12]. K. F. Khaled [13] studied IE of 2-(aminomethyl)benzimidazole (AB), 2-CBI and 2-(methylthio)benzimidazole (MB) for iron in 1M HNO3, and AB shows to be the most effective corrosion inhibitor. By using electrochemical impedance spectroscopy (EIS), R. L. Camacho-Mendoza et al. [14] found that 2-CBI has the highest IE among the six heterocyclic compounds. Z.L. Li et al. [15] found that when benzimidazole and its derivatives are used as corrosion inhibitors for mild steel in 1M HCl solution, the corrosion IE shows a 2-chloromethylbenzimidazole(2-CBI) > 2Methylmercaptobenzimidazole > benzimidazole, where the corrosion IE of 2-CBI can reach nearly 96% at a concentration of 1.52×10−3 mol·L−1. Although 2-CBI has an outstanding IE, it is expensive. In order to expand the application of 2-CBI, to obtain a higher IE with lower 2-CBI concentration is urgent.
Studies [16–18] have found that when a variety of inhibitors were used together, the IE was higher than that of a single inhibitor, and this phenomenon was denoted as synergistic effect. Based on the synergistic effect, one can improve the IE of some inhibitors and reduce the cost of some inhibitors. The mechanism of the synergistic effect was usually ascribed to the interplay between the different inhibitor molecules or the interaction between the corrosion inhibitor and a certain ion existing in the aqueous solution. Researchers have found that chloride ions, bromide ions and iodide ions have better synergistic effects. Among them, due to the largest size and the best polarizability, iodide ions usually has the highest synergistic effect [19–21]. IB Onyeachu et al. [22] found that 5-methyl-1H-benzotriazole (MHB) can inhibit the corrosion of mild steel by 80% in 1 M HCl solution, and the IE of MHB was increased significantly after adding 5 mM potassium iodide into the solution. It was speculated that the iodide anion makes the steel surface negatively charged [23], thereby improving the electrostatic attraction of the protonated MHB on the steel surface. P. Banerjee et al. [24] found that, with an addtion of a very small amount of potassium iodide (6×10−3 mol·L−1), the IE of palmitic imidazole (PI) increases from 90% to about 98% with its concentration of 1.0×10−3 mol·L−1. By using molecular dynamics simulation, they believe the protonated PI may bridge with the previously adsorbed acid anions or iodide anions on the metal surface, thereby reducing the process of H+ gaining electrons and reducing corrosion.
In this contribution, the synergistic effect of 2-CBI/potassium iodide mixture (2-CBI/KI) for mild steel in 1 M HCl was studied. An electrochemical workstation was used to measure the corrosion of mild steel in 1 M HCl solution with different amounts of 2-CBI or 2-CBI/KI with different amounts of potassium iodide. In addition, the adsorption isotherm of 2-CBI/KI on the surface of mild steel was determined, as well as the influence of temperature on the corrosion of mild steel with 2-CBI/KI in the 1 M HCl solution.
2. Experimental
2.1 Materials
2-CBI was purchased from Shanghai Yuanye Biotechnology Co., Ltd.. Potassium iodide and concentrated hydrochloric acid were purchased from Shanghai Runjie Chemical Reagent Co., Ltd., without any further treatment before use. Concentrated hydrochloric acid (37%) was dissolved in distilled water to prepare 1M HCl solution. The chemical composition of the involved mild steel samples is C = 0.193%, Si = 0.272%, Mn = 0.436%, P = 0.033% and S = 0.029%. A cylindrical mild steel sample with a cross-sectional area of 0.785 cm2 was welded with insulated copper wire and sealed by epoxy resin.
2.2 Characterization
A three-electrode cell containing a mild steel sample as the working electrode, saturated calomel electrode (SCE) as the reference electrode, and platinum electrode as the counter electrode was used. An electrochemical workstation (PARSTAT VersaSTAT3) was used to measure EIS and polarization curve of mild steel in 100 mL electrolyte. Before each measurement, the working electrode was immersed in the electrolyte solution for 30 minutes to achieve a steady state. The scanning range of the polarization curve is −250 mV to 250 mV, relative to the open circuit potential (Eoc), with a scanning rate at 1 mV·s−1. EIS measurements were carried out at the Eoc with the frequency range from 105 Hz to 10−2 Hz and amplitude of 5 mV. Each experiment was repeated 3 times and representative results or average values are displayed in graphical or tabular form.
3. Results and Discussion
3.1 Electrochemical analysis
We start with the EIS measurments of the mild steel in 1 M HCl at 20°C with different inhibitors, and the Nyquist plots and Bode diagrams were showed in Fig. 1. The Nyquist plots of mild steel in 1 M HCl with different amount of 2-CBI (Fig. 1a), Potassium Iodide (Fig. 1c) and 2-CBI/KI (Fig. 1e) all show a single depressed capacitive semicircle. The electrical equivalent circuit in Fig. 1a, Fig. 1c and Fig. 1e was used to fit the EIS data and the fitting data were recorded in Table 1, where Rs represents the solution resistance, CPE is the double-layer capacitance and Rct corresponds to charge tranfer resistance.
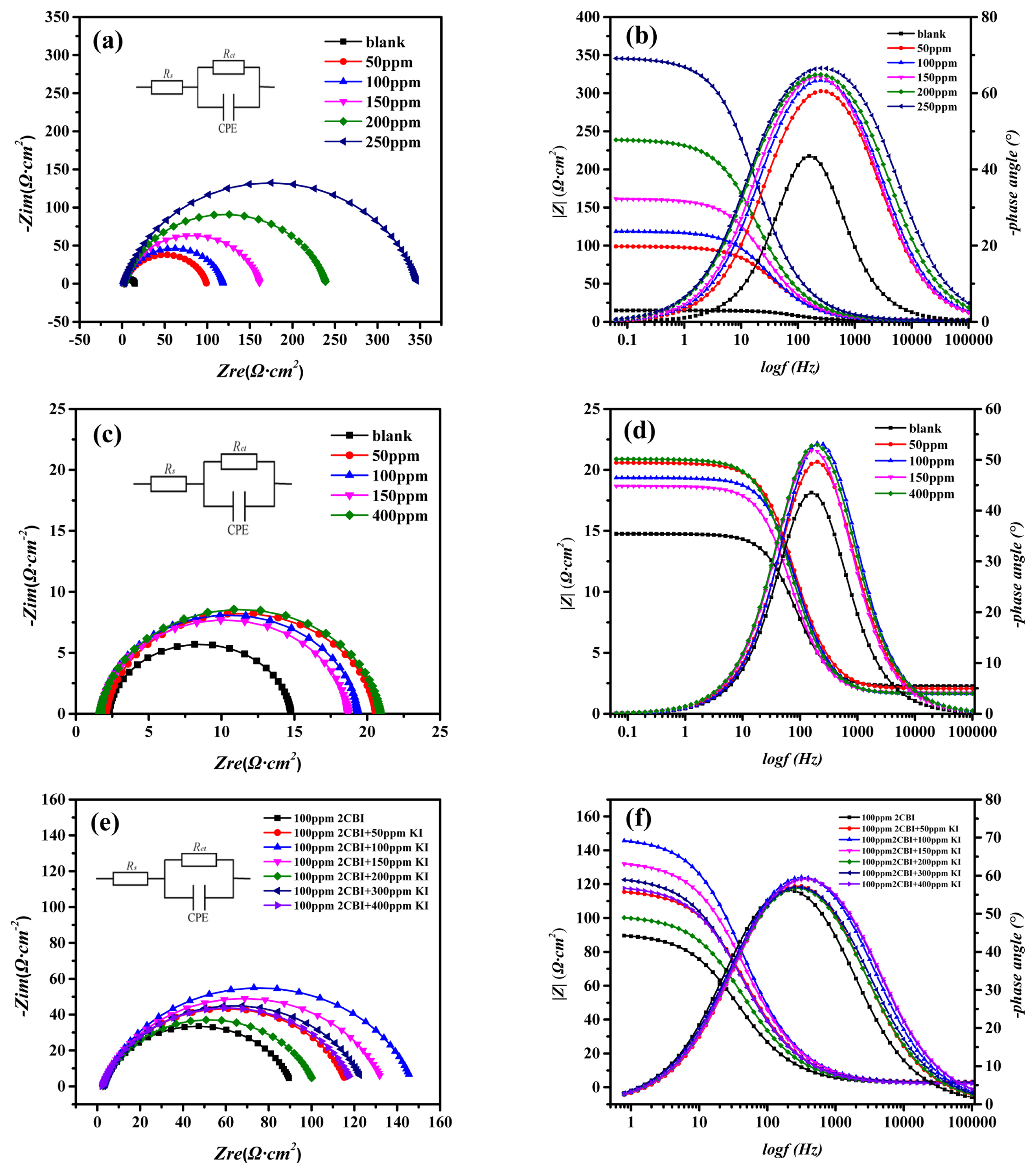
The EIS results of mild steel in 0.1M HCl with different inhibitors at 20°C: the Nyquist plots of (a) 2-CBI, (c) Potassium Iodide, (e) 2-CBI+Potassium Iodide; the Bode plots of (b) 2-CBI, (d) Potassium Iodide, (f) 2-CBI+Potassium Iodide
The IE (η%) was calculated by using the following equations [25]:
where
It can be seen from Fig. 1a that the radius of impedance arc increases with the increasing of 2-CBI amount. From the data in Table 1, it can be seen that as the concentration of 2-CBI increases, the charge transfer resistance Rct increases, indicating that the addition of 2-CBI can form a dense molecular layer on the metal surface and retard the charge transfer process, and thus slow down the corrosion of the metal. From Fig. 1c, one can find that the addition of potassium iodide did not significantly change the radius of the impedance arc. Therefore, it can be deduced that potassium iodide has almost no inhibition effect for the corrosion of mild steel in 1 M HCl at 20°C. However, after adding potassium iodide into 1 M HCl solution with 100 ppm 2-CBI, the radius of the impedance arc changes. It can be seen from Fig. 1e that, with the increasing of potassium iodide amount, the IE of 2-CBI/KI mixture (the concentration of 2-CBI fixed at 100 ppm) increases first and then decreases. And all IE of the 2-CBI/KI mixture are higher than that when 100 ppm 2-CBI was used only.
The Bode diagrams (Fig. 1b, Fig. 1d and Fig. 1f) at low-frequency range confirms the changes of IE with increasing of inhibitor concentration, which depended on the adsorption of the inhibitor on the surface of mild steel [26,27]. It can be seen from the bode phase plot that only one time constant is shown in the figure. Therefore, fitting the EIS data by using the electrical equivalent circuit in Fig. 1a, Fig. 1c and Fig. 1e are reasonable.
Fig. 2 shows the Tafel polarization curves of mild steel in 1 M HCl solutions with different corrosion inhibitors. Corrosion current density (icorr) had been determined by extrapolating the cathode and anode curves to meet the corrosion potential (Ecorr). And the Tafel slopes of the cathode (βc) and anode (βa) were determined. All the parameters obtained from Talfel polarization were listed in Table 2, as well as the surface coverage θ and the IE (η%) calculated by using equation 2 and equation 3 [28,29] respectively:

The polarization curves for mild steel in 0.1M HCl with different inhibitors at 20°C: (a) 2-CBI, (b) Potassium Iodide, (c) 100ppm 2-CBI+ Potassium Iodide
where icorr and
It can be seen from Fig. 2a that, after adding 2-CBI into the 1 M HCl solution, the cathode and anode curves shift to the left obviously, and the corrosion current density decreases greatly. From the data in Table 2, one can find that the IE increases with the increasing of 2-CBI concentration. The results shown in Fig. 2a and Table 2 consist with the results presented in Fig. 1a and Table 1 well, it is easy to say that 2-CBI is an efficient inhibitor in HCl solution solution. After adding different amounts of potassium iodide into the 1 M HCl (Fig. 2b), as indicated in Fig. 1c, the cathode and anode curves have no change, which means potassium iodide has no inhibition effect for mild steel in 1 M HCl. However, as shown in Fig. 2c, both the cathode and anode curves shift to the left after adding different amounts of potassium iodide into the 1 M HCl solution with 2-CBI fixed at 100 ppm. As presented in Table 2, when the potassium iodide concentration is 100 ppm, the corrosion current density decreases from 149.07 μA·cm−2 to 64.82 μA·cm−2, and IE increases from 91.14% to the maximum value of 96.15%. Based on the EIS and Tafel polarization results, it is easy to say, although potassium iodide have no inhibition effect for mild steel in 1 M HCl, but it can enhance the IE of 2-CBI.
According to S.A. Umoren [30], when the halide ions and other molecules were combined and used as inhibitor, there are two mechanisms for their adsorption on the metal surface. One is that the ion pair firstly formed in the solution and then adsorbed to the metal surface. The other is that the halide ions adsorbed onto the metal surface firstly, and then the other molecules were attached by the adsorbed halide ions. Based on the existing researches [31,32], the enhanced IE of 2-CBI by potassium iodide would be ascribed to the following reason that, with potassium iodide in the solution, the first adsorbed I− makes 2-CBI easier to adsorb onto the mild steel surface, and forming a denser molecular layer to protect the mild steel.
Nevertheless, with the increasing of the potassium iodide concentration, the IE 2-CBI/KI mixture reaches the maximum when the potassium iodide concentration is 100 ppm, and then the IE decreases. This probably due to the reason that, as the concentration of potassium iodide further increased, the adsorption of 2-CBI and I− on the metal surface changed from staggered adsorption to overlapping competitive adsorption, thus resulting in a desorption of 2-CBI [33,34].
In a word, combining 2-CBI with potassium iodide in 1 M HCl can induce a higher IE than that of 2-CBI itself, it is safe to say, there is a synergy effect bewteen 2-CBI and potassium iodide when they were used together as inhibitor for mild steel in 1 M HCl. Here, the synergy parameters (S) were calculated by using the following equation [35]:
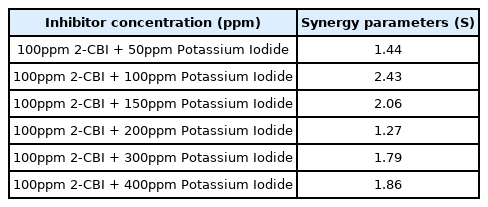
Where θA and θB are the surface coverage of 2-CBI and potassium iodide on the metal surface, respectively, and θAB is the surface coverage of the mixture of 2-CBI and potassium iodide. If S> 1, it indicates synergy, if S <1, it indicates antagonism between 2-CBI and potassium iodide [36]. It can be seen from Table 3 that synergy parameter of 2-CBI/KI mixture are always greater than 1, no matter the concentration of potassium iodide is.
3.2 Adsorption isotherm
The adsorption isotherm can provide information about the interaction between adsorbed molecules and the electrode surface [37]. In order to leran more about how the 2-CBI/KI mixture adsorb on the surface of mild steel, various adsorption isotherm models (such as Temkin, Langmuir and Frumkin models) were used to fit the data. It was found that, for 2-CBI and 2-CBI/KI mixture, the Langmuir adsorption isotherm is working. The calculation results were fitted by the following formulae [38]:
Among them, Kads is the constant of the adsorption-desorption process that occurs on the metal surface, C is the corrosion inhibitor concentration (mol·L−1), and θ is the surface coverage.
The Langmuir adsorption isotherm of 2-CBI and 2-CBI/KI mixture are shown in Fig. 3, respectively. It can be observed that, for the adsorption of 2-CBI or 2-CBI/KI mixture onto mild steel, the correlation coefficient (R2) and slope value are near unity indicating Langmuir adsorption isotherm works very well [39]. The value of Kads was obtained from the intercept of isotherm plot Fig. 3. The calculated values of Kads are displayed in Table 4. The higher the value of Kads, the greater the adsorption capacity and the better the corrosion inhibition effect [40]. The free energy of adsorption
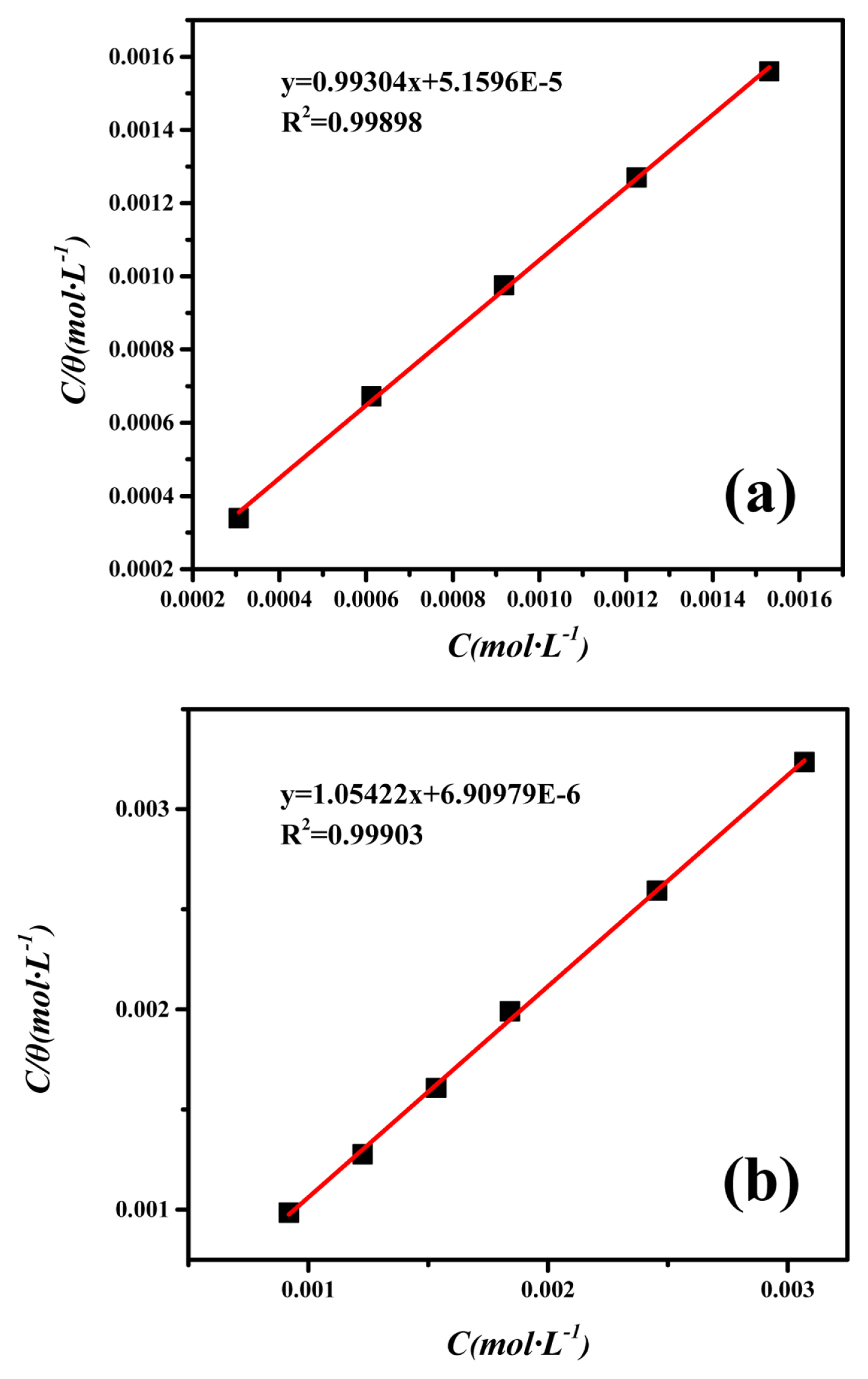
The Langmuir adsorption isotherm of (a) 2-CBI, (b) 2-CBI/Potassium Iodide on mild steel surface in the 1M HCl solution at 20°C
Among them, R is the gas constant, T is the Kelvin temperature, and the value 55.5 represents the molar concentration of water in the acid solution (mol·L−1).
Table 4 shows the Kads and
3.3 Effect of temperature
The effect of temperature [49] on the corrosion of mild steel with inhibitors in the acid solution is very complicated. For example, the anode or cathode reaction, the rapid etching and desorption of the corrosion inhibitor and the decomposition of corrosion inhibitor itself. In order to learn more inhibition mechanism of 2-CBI/KI mixture for mild steel in 1 M HCl, the effect of temperature on electrochemical parameters of mild steel with 100 ppm 2-CBI and 2-CBI/KI mixture (100 ppm 2-CBI + 100 ppm potassium iodide) was performed, the temperature ranging from 20°C to 40°C. Fig. 4 shows Tafel polarization curves of mild steel with 100 ppm 2-CBI and 2-CBI/KI mixture (100 ppm 2-CBI + 100 ppm potassium iodide) in 1 M HCl at different temperatures, and the relevant electrochemical parameters are listed in Table 5.
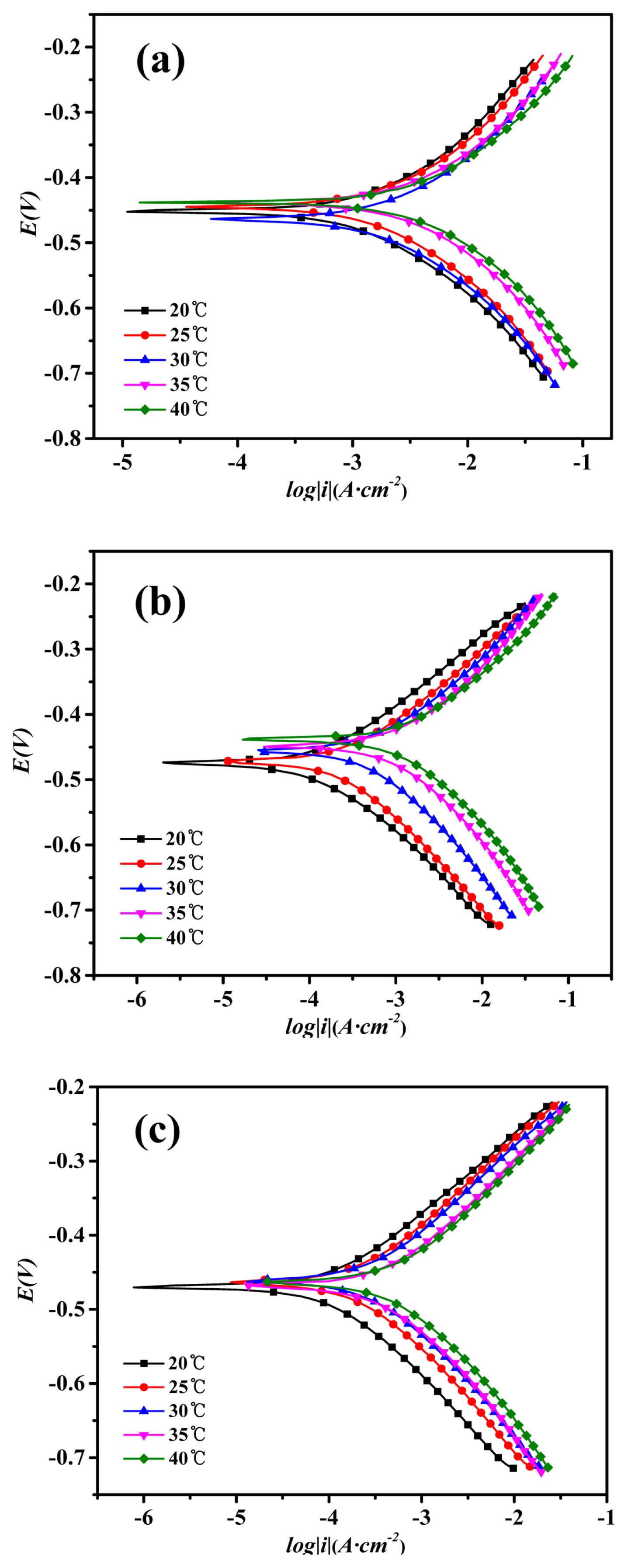
The polarization curves of mild steel in 1M HCl with or without inhibitors at different temperatures: (a) Blank, (b) 2-CBI, (c) 2-CBI+ Potassium Iodide
From Fig. 4 and Table 5, it can be seen that the IE of 2-CBI and 2-CBI/KI mixture both decreases with increasing of the temperature. However, the IE of 2-CBI and 2-CBI/KI mixture response to temperature differently. For 2-CBI, with temperature increasing from 20 to 40°C, the IE decreases from 92.41% to 72.20%. While for 2-CBI/KI mixture, the IE decreases from 93.39% to 89.17%. The smaller IE reduction with temperature increasing indicates that the 2-CBI/KI mixture molecule film has the higher stability than that of 2-CBI alone.
Further, the activation energy of the corrosion process was calculated by using Arrhenius and transition state equation [50–52]:
Among them, A is the pre-exponential factor, R is the gas constant, h is the Plank constant, N is the Avogadro number, Ea is the apparent activation energy, ΔSa is the entropy of activation, and ΔHa is the enthalpy of activation respectively.
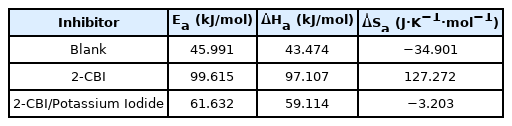
The plots (Arrhenius plots and transition state plots) for mild steel in 1M HCl solution witout inhibitor or with different inhibitors are shown in Fig. 5 and the relevant data are listed in Table 6. One can find that, without any inhibitor, the value of Ea is 45.991 kJ·mol−1, while with 2-CBI or 2-CBI/KI mixture as inhibitor, the value of Ea is 99.615 kJ·mol−1 and 61.632 kJ·mol−1 respectively. According to the exsiting researches, the increasing of Ea means the interaction between the inhibitor and the metal surface more inclined to physical adsorption, while the decreasing of Ea is more inclined to chemical adsorption [53,54]. Those results can also confirm the fact that, potassium iodide can promote the chemisorption of 2-CBI on the mild steel surface in 1 M HCl.
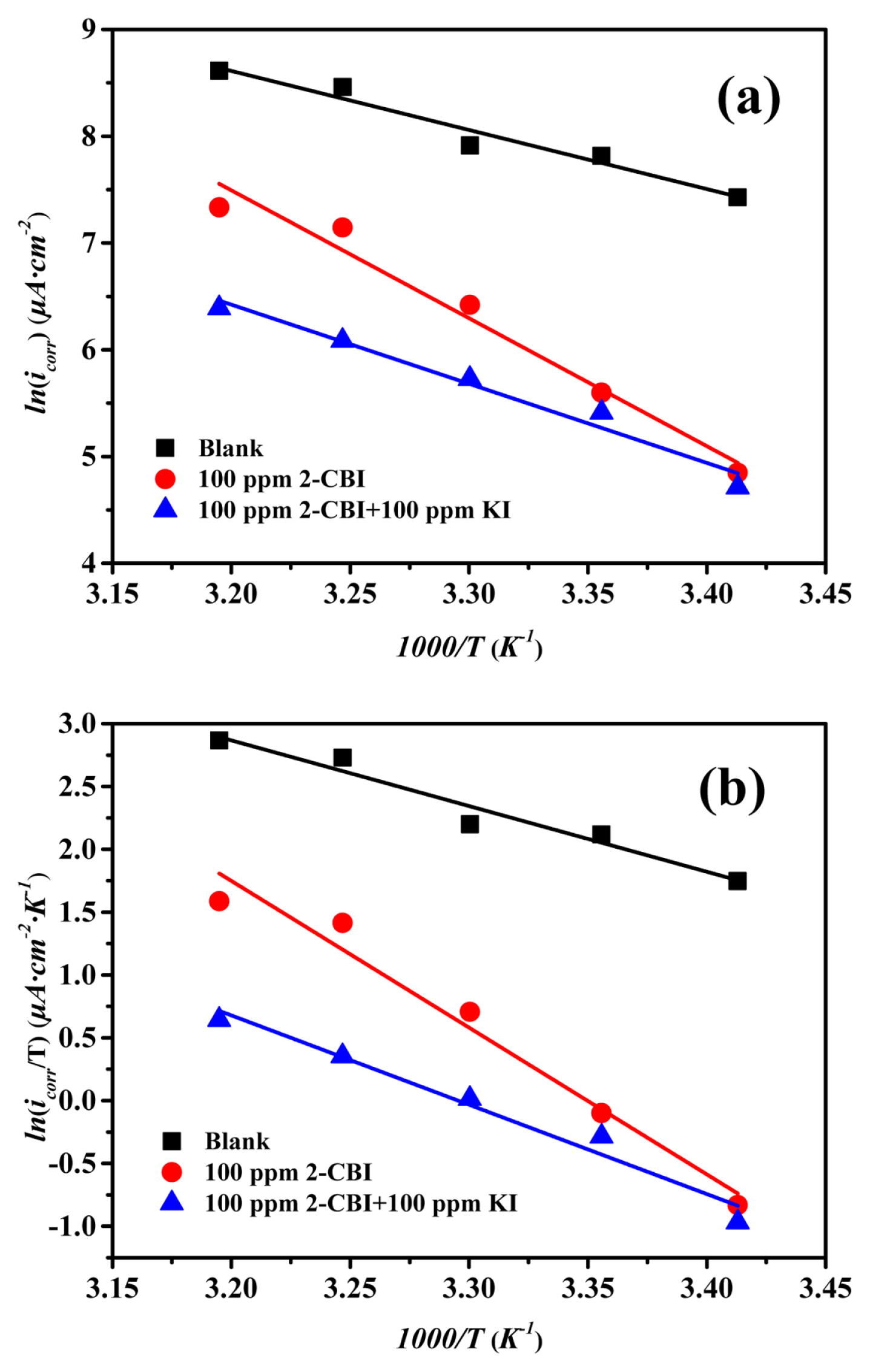
Arrhenius plots (a) and transition state plots (b) for mild steel in 1M HCl solution without and with different inhibitors
In Fig. 5 and Table 6, there is a good linear relationship between lnicorr and 1000/T as well as ln (icorr/T) and 1000/T. It can be observed from Table 6 that the Ea has the same trend with ΔHa, which can be explained by the equation,ΔHa= Ea-RT. In addition, the apparent entropy change (ΔSa) with 2-CBI as inhibitor is greater than that of the control. And this phenomenon can be ascribed to the the competitive adsorption of water molecules and corrosion inhibitors. However, the addition of potassium iodide reduces the value of ΔSa sharply, which probably means that potassium iodide promoted the chemisorption of 2-CBI on the surface of mild steel and thus form a more stable2-CBI film to ptotect the mild steel [55].
4. Conclusions
The synergistic effect of 2-CBI and potassium iodide for mild steel in 1 M hydrochloric acid solution was proved to be effective by electrochemical test. Although potassium iodide have no inhibition effect for mild steel in 1 M HCl, but it can enhance the IE of 2-CBI and the synergistic parameter of 2-CBI/KI mixture (100 ppm 2-CBI with different amount of potassium iodide) is always greater than 1.
Langmuir adsorption isotherm is working in this experiment, the
Based on the adsorption isotherm results and the effect of temperature, it is easy to say, after adding potassium iodide, the interaction between inhibitor and the surface of mild steel changes from physical adsorption to chemisorption. With potassium iodide in the solution, the first adsorbed I− makes 2-CBI easier to adsorb onto the mild steel surface, and forming a denser molecular layer to protect the mild steel.
Acknowledgements
The financial support from National Natural Science Foundations of China (No. 51903111), the Founding for Talent launching of Jiangsu University of Science and Technology (1062931603), and the Jiangsu Undergraduate Innovation and Entrepreneurship Training Program (2021) are appreciated.