A Techno-Economic Study of Commercial Electrochemical CO2 Reduction into Diesel Fuel and Formic Acid
Article information
Abstract
The electrochemical CO2 reduction (ECR) to produce value-added fuels and chemicals using clean energy sources (like solar and wind) is a promising technology to neutralize the carbon cycle and reproduce the fuels. Presently, the ECR has been the most attractive route to produce carbon-building blocks that have growing global production and high market demand. The electrochemical CO2 reduction could be extensively implemented if it produces valuable products at those costs which are financially competitive with the present market prices. Herein, the electrochemical conversion of CO2 obtained from flue gases of a power plant to produce diesel and formic acid using a consistent techno-economic approach is presented. The first scenario analyzed the production of diesel fuel which was formed through Fischer-Tropsch processing of CO (obtained through electroreduction of CO2) and hydrogen, while in the second scenario, direct electrochemical CO2 reduction to formic acid was considered. As per the base case assumptions extracted from the previous outstanding research studies, both processes weren’t competitive with the existing fuel prices, indicating that high electrochemical (EC) cell capital cost was the main limiting component. The diesel fuel production was predicted as the best route for the cost-effective production of fuels under conceivable optimistic case assumptions, and the formic acid was found to be costly in terms of stored energy contents and has a facile production mechanism at those costs which are financially competitive with its bulk market price. In both processes, the liquid product cost was greatly affected by the parameters affecting the EC cell capital expenses, such as cost concerning the electrode area, faradaic efficiency, and current density.
1. Introduction
The continuous consumption of fossil fuel to meet the 82% global energy demands has increased the CO2 concentration in the atmosphere (412 ppm) and these large concentrations have caused detrimental impacts on the environment and are a serious threat to the ecological balance and the living community [1,2]. It is still a great challenge to reduce CO2 concentrations globally because the world population and energy demand are predicted to increase. Nevertheless, renewable energy means like solar and wind are getting more market attention and share, fossil fuels will remain prime energy means up to the midcentury. The dependence of the chemical and transportation sectors on traditional natural resources is the main reason behind it. For instance, it is estimated that the contribution of renewable resource in electricity generation will increase to around 27% by 2040, the use of renewable energy in chemical and transportation sector will be 9% and less than one percent, respectively [3]. Because petroleum-derived automobiles will remain economically dominant and traditional fuels will stay crucial feedstock for the next decade.
Among the CO2 mitigating technologies, CO2 conversion is an attractive approach that could reduce the global energy crisis by transmuting CO2 into value-added chemical molecules and energy fuels using renewable energy means [5–7]. Presently, CO2 conversion can be divided into electrochemical, photochemical, and thermochemical technologies. The electrochemical CO2 reduction (ECR) integrated with renewable energy means (to obtain electricity) is widely utilized in chemical and energy sectors and could offer a promising route to produce important carbon-neutral fuels and chemicals. Compared to others, electrochemical CO2 conversion has various benefits: (1) the formation of hydrocarbons from renewable electricity, carbon dioxide and water can be accomplished; (2) the transmutation systems are scalable, on-demand, highly efficient and compact; (3) the mechanism is easier and precise to administer by simply monitoring the reaction temperatures and electrode potentials; (4) the operation can use clean energy means like tidal, geothermal, wind and solar plus additional electricity obtained from hydroelectric and nuclear sources [8]. By using this technology, carbon-neutral electricity means can be employed to electroreduce CO2 to produce useful chemicals and fuels, therefore closing the carbon cycle and mitigating CO2 emissions. The achievements to date in ECR technology are very promising and scientific community is paying much attention towards its development. Most research studies have discussed the reaction mechanism of electrocatalysts and their underlying working principles, whereas others have concentrated on the improvement of bench-scale EC cells for efficient CO2 reduction [7]. Nevertheless, very few research studies have been reported to comprehend the viability of ECR technology as a way of producing chemicals and fuels on techno-economic grounds and what factors can affect its commercial-scale utilization. Few techno-economic reports on electrochemical CO2 reduction mechanism have been previously reported [9–13]. For instance, Jonggeol et al. reported a techno-economic analysis of electrochemical CO2RR organic oxidation reaction coproduction through conceptual process design and therefore proposed a series of possible potential economic combinations [30]. They performed above 290 combinations of electrochemical coproduction to analyze the techno-economic viability of ECR technology to discover a potential combination. A fabrication framework was developed to study a variety of coproduction processes whereby all the factors impacting the production cost, including recycling systems, separation processes, electrolyzer systems, and various utility systems, were considered, hence securing analytical reliability.
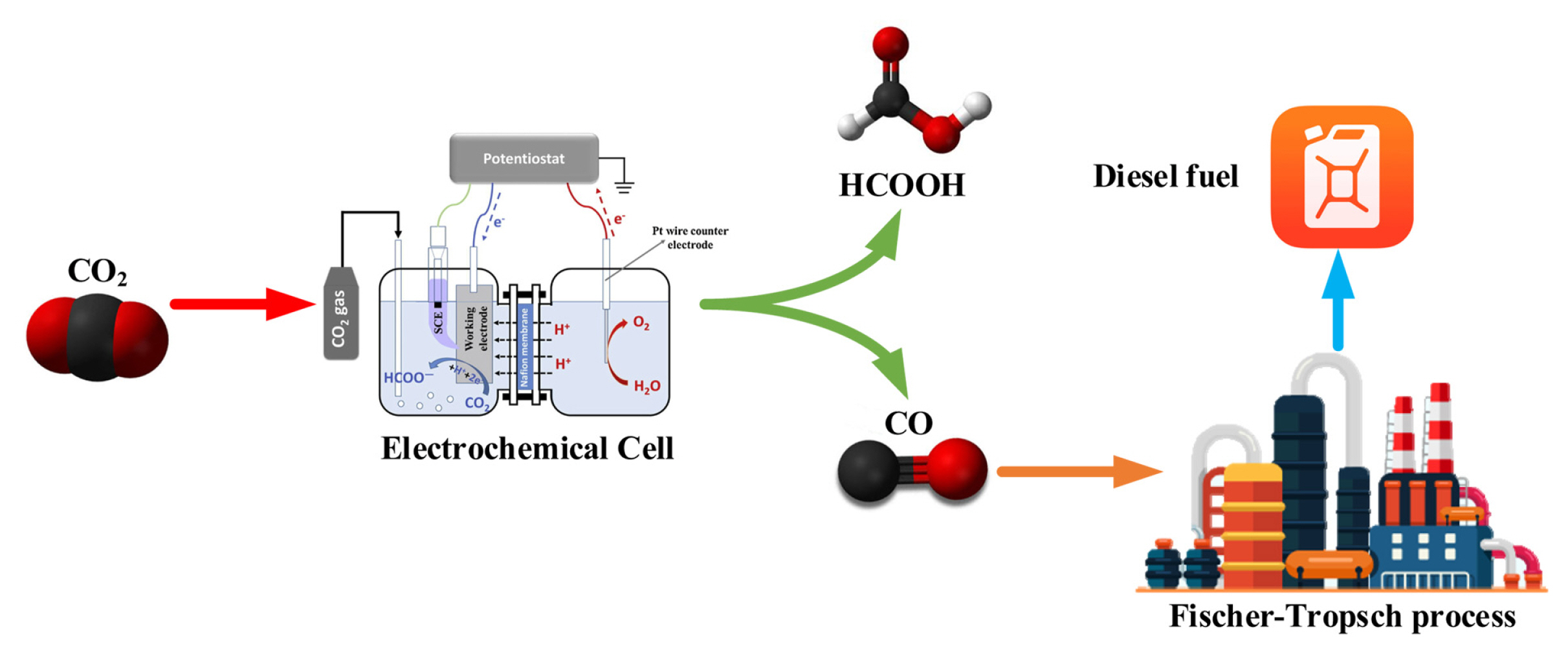
The conversion of CO2 into a liquid product having high energy density will be most desirable because they can be conveniently transported and stored, and utilized on-demand for high energy applications. Herein, the production of diesel fuel and formic acid through electrochemical CO2 reduction using a consistent techno-economic approach is presented. The process diagram is shown in Fig. 1. Established techno-economic assessment methodology was used to find the economic viability of these two processes. Diesel fuel is not directly produced through ECR, but produced by syngas (CO+H2) conversion through Fischer-Tropsch process [14,15]. In the first case, CO needed to produce diesel fuel was obtained from ECR, and this process is indicated as CO2-CO-diesel fuel. For the second case, the direct CO2 electroreduction into formic acid was analyzed. The electrochemical CO2 reduction to produce CO with high faradaic efficiency at small overpotentials has already been reported [16]. Similarly, formic acid production has also been displayed achieving high stability and faradaic efficiency, though at the expense of high overpotential [17].
2. Techno-economic assumptions of system elements
2.1 Power plant
A power plant of 500 MW capacity is considered for the present techno-economic study and all the CO2 needed for the ECR process was obtained from the flue gases of this power plant [15]. Using no any carbon capture mechanism, most of the coal-derived power plants discharges CO2 at a rate of 830 kg/MWh [18]. The operating and capital costs associated with power plant are included in terms of electricity prices bought from the grid instead of directly incorporating them in the economics of CO2 reduction analysis.
2.2 CO2 capture
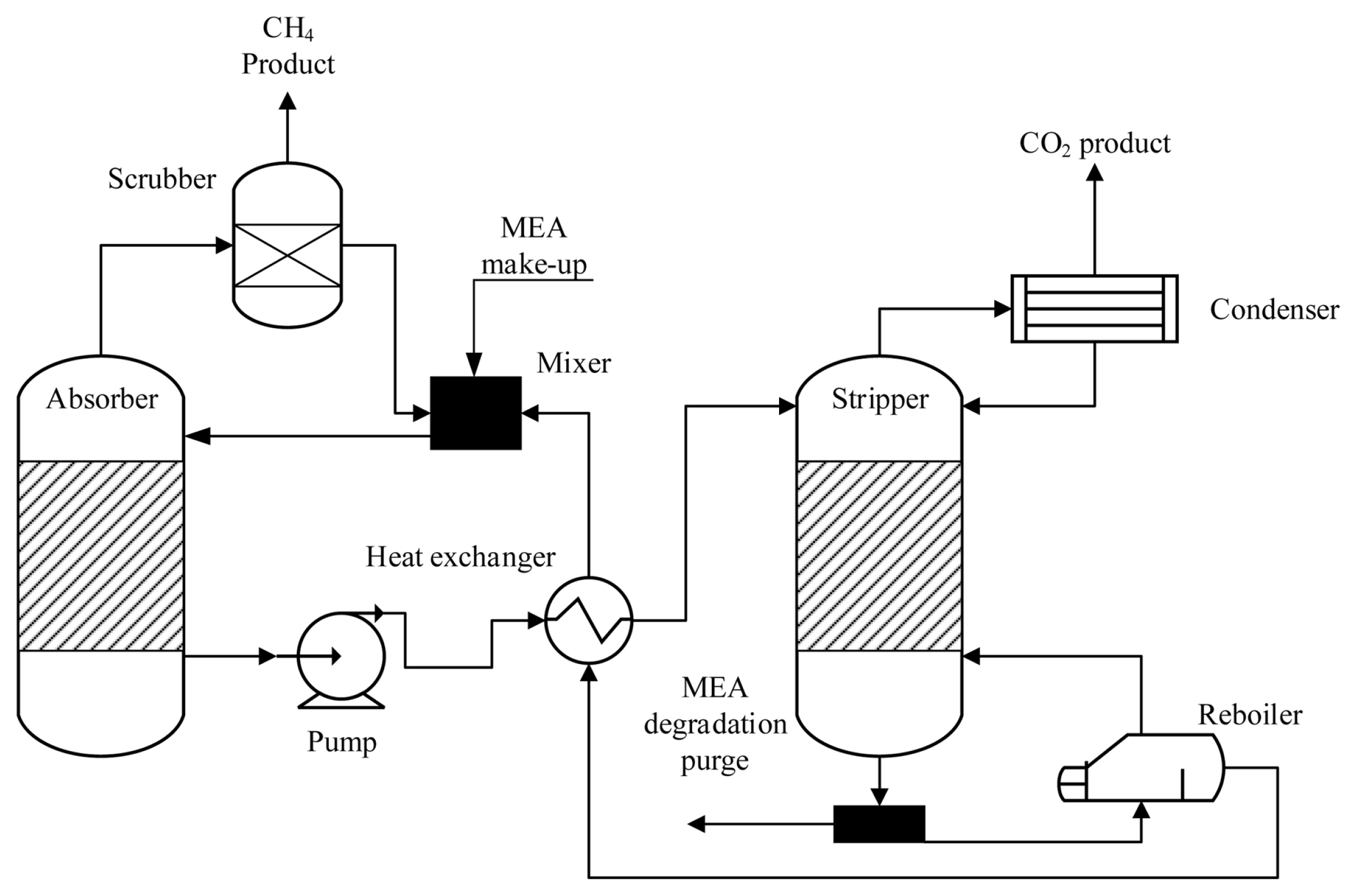
An efficient carbon capture mechanism is needed to draw out concentrated CO2 from the exhaust of the power plant. In Fig. 2, the process of CO2 capturing is illustrated. Among the presently adopted CO2 capture technologies, regenerative systems incorporating amine-based solvents have been considered the most appropriate approach that is being used on a commercial scale. In this approach, the mixture of MEA solution and biogas is fed into an absorption column where it reacts with CO2 to produce a soluble salt.
The CO2 is discharged from the absorber, and MEA solution enriched with CO2 is supplied to a heat exchanger where the temperature of the solution is increased to 120°C and then sent to a stripping column. MEA is reproduced in the stripping column and forwarded to the absorber for reutilization. The reproduction conditions are sustained by the boiler using low-pressure stream, which operates as stripping fluid in the column and is regenerated by the condenser and sent back to the stripping column, whereas the stream of concentrated CO2 is discharged from the upper side of the stripping column for downstream operation. The operating and capital expenses for the CO2 capture mechanism were incorporated in the present study, and average CO2 discharges at a rate of 121 kg/MWh were recorded when an efficient CO2 capture system was employed, transferring 719 kg/MWh of CO2 for ECR. The carbon capture mechanism is observed to reduce the power production overall efficiency by 20–25%, and we have accounted 25% decrease in the operating expenses of the carbon capture mechanism in terms of electricity demand per year. The important features of CO2 capture are enlisted in Table S1.
2.3 Electrochemical (EC) cell
This is the extremely important constituent of the ECR, and its performance is measured by Faradaic efficiency (FE), current density (CD), and the overpotential. FE describes the selective formation of different hydrocarbons during ECR and basically designates the ratio of total charge transferred being utilized to produce a certain product. This index is used to measure the selectivity of a certain product during ECR. High faradaic efficiency is needed to reduce the important separation processes that could greatly enhance the overall operational and capital costs. CD illustrates the ratio of current flow to active electrode area at a certain potential. In other words, the current density is the quantification of the EC reaction rate per electrode area and is utilized to measure the overall electrode size required to get the desired reaction rate. Moreover, different factors like reactant and products delivery rate, catalyst utilization, and catalyst loading affect the current density. Overpotential is the difference between thermodynamic potential, and in ECR, anodic OER and cathodic reduction reaction demonstrate the considerable anodic and cathodic overpotential, respectively. It was found that overall overpotential stays higher than the sum of both overpotentials since large current density directs to the ohmic loss in the cell. The catalyst’s ability to decrease the reaction barriers for ECR can be estimated when the overpotential values are compared at the same current density and faradaic efficiency.
ECR is similar to water electrolysis in several aspects, and therefore EC cell has many common design characteristics. However, the cathode and membrane material are different, industrial EC cells are therefore quite identical to PEM water electrolyzers having similar construction and constituents [20]. The generally employed PEM models to reduce water into hydrogen were used to evaluate and assess the capital costs for EC cell. To estimate the capital expenses of EC cell, PEM electrolysis reference parameters were employed and has been enlisted in Table S2 [21]. The inflation-accommodated uninstalled EC cell expenses were $30.9×103 per square meters after incorporating the balance of plant (BoP) and stack costs. The different expenses such as site preparation and contingency (given in Table S2) constituting capital costs per square meters are not accounted for EC cell downtime for overhaul and maintenance and therefore 0.97 capacity factor for EC cell was considered when calculating the overall electrode area needed to address the production rate per year [21,22]. The overall electrode area needed was calculated from the faradaic efficiency, EC cell current density, and production rate of liquid products. Replacement costs of the important components every 7th year were evaluated at fifteen percent of the EC cell installed capital cost. Therefore, the stability of EC cell components is assumed for the base case assumptions for CO2 electroreduction into CO in the present analysis, and ECR catalysts correspond to the replacement proportion. The 50% single-pass CO2 transmutation in the EC cell was considered for the base case assumption and was greatly affected by the EC cell design [15]. The statistics utilized herein demonstrate the complete capital expenses for a PEM electrolyzer and were derived from the H2A model [21]. The cost/electrode area for the base case assumptions considers a relatively higher scaling factor and linear scaling, causing a decrease in the capital cost. In this techno-economic analysis, decreased costs attributed to scaling are restricted to those parameters used in optimistic case assumptions and sensitivity analysis.
2.4 Electric power
The electric power needed to operate the EC cell and auxiliary equipment for ECR greatly increases the operating expenses per year. However, its price can change by source and region versus industrial applications across the globe. The average price of electric power for base case assumptions was taken as $0.05/kWh. The cost of electric power to produce H2 through electrolysis for Fischer-Tropsch process is implied in the dollar per kg cost of the hydrogen and therefore wasn’t included in the electric power consumption per year. The expenses of CO2 capture were added in this study by considering the capital costs of CO2 capture and including the extra electric power consumption needed to operate the CO2 capture mechanism to operating costs per year (as mentioned in Table SI). Using the wind-generated or solar photovoltaic electric power to operate the ECR will greatly help in mitigating the large CO2 concentrations and the electric power cost obtained from such renewable resources is highly competitive and in several parts of the world already similar to the price of electric power harnessed from the fossil-fuel [23].
2.5 Product separation
Since unconverted CO2 reactants pass through the EC cell, it is highly beneficial to isolate non-reacted CO2 from the final gas products and reuse it in the EC cell. In such a way, not only most of the CO2 can undergo a reduction process, but carbon capture costs can be significantly reduced with respect to the rate of product production. To achieve cost-efficient commercial separation of final products, pressure swing adsorption (PSA) is a state-of-the-art approach extensively used for the purification of gases [15], and it has also been displayed for CO2 separation from carbon monoxide. In Table S3, capital costs are enlisted for a reference recycle arrangement utilized to estimate the flow rate of different gases leaving the EC cell of the modeled CO2 conversion system. It was considered that the separation process allowed 97% of the carbon dioxide to be eliminated from the EC cell output and resent to the input section with very little amounts of ethylene, hydrogen, and carbon monoxide.
2.6 Carbon credits
The electroreduction of CO2 reduction to produce valuable chemicals and fuels is a promising technology to mitigate GHG discharges. The CO2 reduction will be financially important if taxes are imposed on CO2 emissions, and this policy is being practiced in many countries across the globe. The importance of CO2 mitigation in such operations could be lucrative if a cap and trade strategy in which ECR technology could sell out carbon credits to different industries. Currently, many countries don’t have any trading scheme or emission taxes, and either of them can’t be implemented in the near future. The base case assumptions in the present analysis considered $0/ MT of CO2 sequestered into value-added products, but a high price of hundred dollars for each metric ton of carbon dioxide was accounted for the sensitivity assessment. The amount of CO2 sequestered per year in CO2 electroreduction to CO was measured depending upon the relevant carbon molecules in each chemical, molar rates of byproduct and the liquid product. The above discussed carbon credit was assumed as a counterbalance for the operating expenses per year.
2.7 Maintenance cost and byproduct value
Based on previous studies [24,25], 3.2% annual maintenance and operating expense were employed for the installed capital expense of the EC cell. The same percentage was employed to overall capital costs of the Fischer-Tropsch process, gas separation, and CO2 capture to add their maintenance and operating expenses. Having superior faradaic efficiency for ECR to produce certain products, considerable amounts of byproducts were formed. The processing of CO in Fischer-Tropsch process involves their combustion to generate the electric power credit.
2.8 Production of liquid fuel
The carbon monoxide produced from ECR was integrated with a separate stream of hydrogen to generate syngas (CO+H2) which is a key feedstock for diesel formation through Fischer-Tropsch process. Since the ECR is supposed to be completely carbonfree, the hydrogen stream was considered to be taken from water electrolysis derived by clean energy resources, and $5/kg hydrogen cost was considered [22]. The other parameters for diesel production through Fischer-Tropsch process in this study were taken from [14], which discussed the coal transformation into diesel. In Table S4, the breakdown of energy consumption and capital costs by system constituents for the model Fischer-Tropsch process is shown. This model process was actually a transformation of coal into electricity and diesel fuel using recycled untransformed syngas [14]. Nevertheless, this model Fischer-Tropsch process had many essential components for coal synthesis and its transformation into syngas. All of these components are not needed for the production of diesel liquid when EC cell is used to produce carbon monoxide. The down-stream system constituents used for syngas processing were considered in the present study. The diesel was distillated in this model Fischer-Tropsch process and the carbonaceous commodities were burnt to produce the additional electric power, which was further used in Fischer-Tropsch process to meet the electricity demand. For the sensitivity assessment on the impacts of changes in the Fischer-Tropsch transformation rate, the electric power produced by the combustion of carbonaceous commodities was proportionately accommodated. Corresponding to the reference system, a 34% conversion of carbon monoxide into diesel through Fischer-Tropsch process was considered.
3. Methodology
3.1 Analysis of net present value (NPV)
For both processes, an absolute discounted cash flow assessment was utilized for the plant lifetime of 20 years to identify a Levelized fuel cost (LFC) for every obtained liquid product. The capital cost of each plant facility was considered sustained during a construction period of one year, whereas the product revenue (PR) and operating cost (OC) were produced every year for plant life time (20 years) and were deducted from the construction year utilizing 12% absolute discount rate (dr) as per the following mathematical expressions [30]:
A pre-tax environment was assumed, and capital assets depreciation was not incorporated in the calculations. Substitution expenses for electrolyzer components were also added as operating costs every six years. The LFC for a particular product in each route was calculated by:
3.2 Cost assessment
Both systems for CO2 electroreduction plant utilized the base-case design conditions, and financial parameters are given in supporting information. Each system was designed to administer the emissions from a 500 MW coal-derived electric plant as indicated in the section techno-economic assumptions. In each case, the capital components and system were supposed to operate for a period of 20 years. All results and capital expenses were accommodated with respect to relevant source data to consider the inflation and developed to 2020 dollar estimates.
4. Results and Discussion
Based on the assumptions mentioned in the above sections, we have performed the techno-economic feasibility of diesel production from CO produced through electrochemical CO2 reduction (ECR). The parameters for each respective base case are provided in the supporting information. Corresponding to the base case assumptions, a common process flow chart having molar flow rates is incorporated. These statistics were further utilized to calculate the product revenue, operating, and capital costs which were afterward employed to estimate the product LCF as per equation 3. To determine the impacts of changes on the crucial system constituents, sensitivity analyses for the process were carried out. To understand the cost reductions with respect to system improvements for diesel production, optimistic assumptions were used concurrently, and the obtained optimistic LCF was compared with the base case assumptions. The optimistic assumptions were further improved by considering a supportive policy structure in terms of monetary values for the carbon discharges mitigated by diesel production.
4.1 CO2-CO-diesel fuel
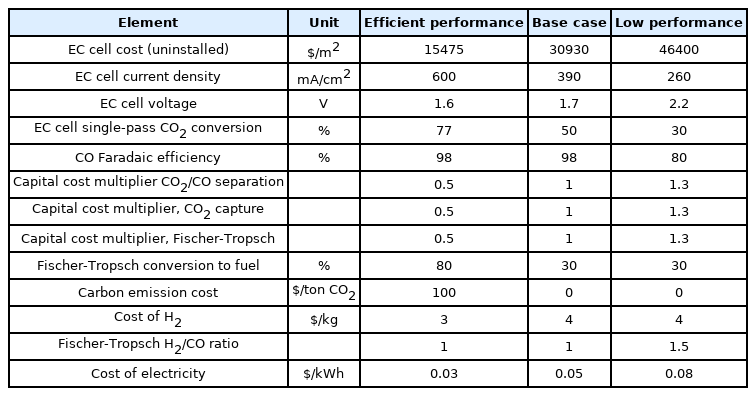
Different assumptions for CO2-CO-diesel fuel are outlined in Table 1. It should be noted that the production of diesel fuel through Fischer-Tropsch approach, which actually comprised of hydrocarbons mixture, was considered as C10H22 to find out the molar flow rates. Base case values considered an efficient EC cell for the production of CO, and 34% base case conversion of CO to diesel was assumed as per the reference [14].
Although, some studies [14,15,26] have reported this conversion rate up to 80% and therefore considered for the superior performance in Table 1. The multiplier parameters for capital expense consider an increase or reduction in the overall capital costs of the integrated system constituents. The other system metrics for CO2-CO-diesel are provided in Table S5.
4.1.1 Sensitivity analysis
Fig. 3 depicts the sensitivity analysis of low and high-performance parameters and their impact on the obtained LCF for liquid fuel (diesel) with respect to the costs of commercially produced diesel. The diesel commercial price changes across the globe and lies between $2.50–3.70 per gallon, and calculating the energy density difference between diesel fuel and gasoline, the market price comes to be $2.22–3.29 per gallon of gas equivalent (GGE). Therefore, the base case assumption will not be financially suitable without making necessary modifications.
The single-pass CO2 reduction to carbon monoxide in the EC cell slightly affected the LCF since CO2 separation and reuse mechanisms were supposed to convert the most amount of CO2. The CO2 capture, product separation, and FTP collectively constituted 25% of the overall base case assumption capital cost, and therefore improvement in capital expenses didn’t cause great alterations in the LCF of diesel fuel. Among the parameters given in Table 1 for sensitivity analysis, the feasible enhance in the Fischer-Tropsch transformation efficiency displayed the highest reduction ($10.7/GGE) in the LCF of diesel fuel for the superior performance case. The superior performance case assumed a steady transformation rate derived from the research investigations, causing a great enhance in base-case assumptions from the model/reference Fischer-Tropsch literature. Costs can be significantly lowered by enhancing the Fischer-Tropsch transformation since more fuel could be produced by electro-converting CO2. A superior faradaic efficiency of the certain products is similarly important in enhancing the EC cell capital investment but in CO2-CO-diesel fuel process, it is (98%) negligible. A notable variable was electric power cost. CO2 capture and electrochemical reduction require high energy amounts, making CO2-COdiesel fuel process a highly energy-intensive process. Although, reducing the electric power price is difficult and needs alterations through the industry. The improvements in the EC cell parameters can be considered an attractive opportunity to reduce the costs of CO2-CO-diesel fuel process. However, lowering the cell voltage reduced the LCF by lowering the EC cell current density and electric power usage. Capital expense with respect to the area of the electrode was also a notable element. Reduced current density indicates that a high overall area of the electrode is needed for a certain CO formation rate, which will increase the EC cell capital expense. Decreasing the EC cell expense in terms of electrode area by design modifications can greatly save the EC cell capital expense by approximately 80% of the capital expense of the overall base case, as shown in Table S5. An important component to increase the operating costs is the amount of hydrogen required to mix the obtained CO into syngas for FT transformation. At a reasonably low expense of $2/kg for purified hydrogen, the LCF of diesel was reduced by $1.6/GGE. In the base case assumptions, ratio for hydrogen and carbon monoxide was selected 1:1 considering it appropriate for diesel formation, but in low-performance assumptions where 2:1 was required, the LCF experienced a considerable increase of 6.7/GGE. Therefore, the selection of appropriate FT reactor and catalyst to achieve high performance having less H2 is quite critical for the system cost. If FECO of EC cell is 50% decreased having the water-splitting balance, then carbon monoxide and hydrogen would be mutually evolved at required ratios for the FT conversion, requiring no extra hydrogen. Nevertheless, the combined formation of syngas constituents was expected to enhance the LCF of diesel fuel to an overall amount of $26.8/GGE, and the combined formation of CO and H2 didn’t affect the total expenses as the equal current must move to form similar molar flows of syngas.
Employing base case assumption expenses ($23.4), improvements in several system constituents are required to enhance the possibility of the economic viability of the CO2-CO-diesel fuel process. These assumptions resulted in the optimistic case with diesel fuel LCF $6.3/GGE, keeping the FT process well close to the industrial viability. The diesel fuel cost was reduced to $4.6/GGE when the carbon emission discharges with an advantage of hundred dollars per CO2 ton were employed. With the decrease in LCF, the financial advantage of carbon emission costs becomes very obvious and can generate a difference between impractical and feasible technology.
5. Electrochemical CO2 reduction into HCOOH
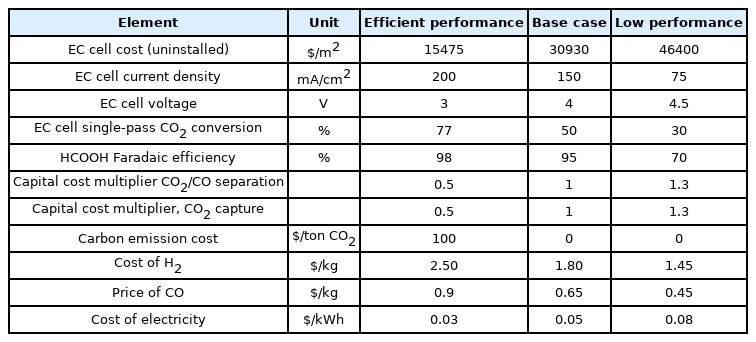
For the present techno-economic study, the second product considered was the formic acid (FA) which is extensively used in hydrogen storage applications and has low volatility and considerable stability at room temperature [27–30]. It is not energy-intensive like other ECR products such ethanol or diesel fuel. The base case assumptions for EC cell were derived from an efficient FA electrochemical reactor [20] employing Sn nanoparticles accompanied by a new multi-constituent ion transfer membrane. This study reported 94% FEFA at a potential of 140 mA/cm2, however, the needed cell voltage at 3.5 V was comparatively higher than the other systems. The base case assumptions for formic acid production through ECR are tabulated in Table S9. An LCF of $3.4 per kilogram and energy density of $31.4/GGE for FA was obtained when base case values were employed [27]. It is obvious that FA formed at this market value will be less financially viable compared to gasoline. However, FA in the form of a chemical is traded at almost $0.6 per kilogram, therefore, although the base case assumptions for FA show that its production as a fuel is not feasible, ECR to FA could be financially competitive in the chemical market. Dissimilar to FT-process, the FA LCF is incorporated in sensitivity assessment and optimistic scenario in dollar per kilogram in order to compare with the market price.
5.1 Sensitivity analysis
A sensitivity analysis (as shown in Fig. 4) was performed based on the high and low-performance values provided in Table 2. The impacts of CO2 transformation rate, byproduct expenses, and non-EC cell capital costs were not high. Lowering the applied EC cell potential only decreased the costs by reducing the electric power consumption of the EC cell. A reduction of 0.5 V from the base case assumption resulted in the superior performance only lowered the overall electric power usage by 14%. The capital cost of the EC cell units was expected the major cost. Therefore, first-hand decrease in electrode area costs had a great impact, reducing 50% dollar per square meter resulted in $0.53 per kilogram drop in LCF. For a base case FE of 94%, the improvement space was little, even a little decrease in low performance significantly enhanced the dollar per kg cost of FA. Nevertheless, the applied cell voltage was high for FA production compared to CO formation, and it didn’t significantly affect the performance values. The current density was also equally crucial, LCF experienced a $0.42/kg decrease when the current density was 50% increased.
Therefore, it is important to maintain the EC cell current density for acquiring economic feasibility. The FA expense was $0.64 per kilogram when the optimistic assumptions were used, keeping it in the price range of industrial HCOOH. This advantage is fundamentally attributed to the reduction in EC cell cost per area and enhance of assumed current density.
Hence, with EC cell design modifications to achieve elevated current densities and EC cell constituents expense reductions and advantages from industrial scale-up, the CO2 electroreduction to produce formic acid might be economically viable. Furthermore, considering the $100/ton carbon emission expense further reduced the FA expense to $0.48 per kilogram, being cheaper compared to the traditional market price.
6. Future Perspectives
This study analyzed the electrochemical CO2 reduction to carbon monoxide (further processed to diesel fuel) and formic acid utilizing a consistent techno-economic approach and excellent assumptions for several ECR conditions. Although the practically obtainable commercial statistics may vary from the base case assumptions, the behaviour of product cost and associated sensitivity of parameters for the two systems demonstrate that these processes are techno-economically feasible. It was observed that the capital cost of EC cell in both cases was quite important. Thus, efforts to lower the EC cell cost with respect to the electrode area and improve the current density are crucial. The routes to achieve this involve modification of EC cell to enhance the amount of CO2 available for the catalyst and cheap auxiliary constituents. Capital costs can also be effectively lowered if EC cell integrated with membranes are used at a commercial scale. The high faradaic efficiency of desired products can also help lower capital costs and surplus energy utilization. On the other hand, reducing the cell voltages was found to slightly reduce the costs, hence it is a secondary concern towards cost-effective ECR. Therefore, the scientific community engaged in ECR investigations may get more advantages if attention is paid towards enhanced current density and product selectivity despite high overpotentials are needed. The expected LCFs for both systems under different conditions are provided in Fig. 5.

Fuel summary for both processes with respect to their base, optimistic, and optimistic plus hundred dollars per CO2 ton
From the perspective of forming a cost-efficient fuel through ECR, the production of diesel fuel is more promising, having LCF of $6.3/GGE. Nevertheless, this scheme needed considerable improvement in the EC cell and FT transformation rates. The present techno-economic analysis doesn’t consider the size of the market. The fuel market is quite big and improbable to restrict the analysis, but the HCOOH market is well smaller and can influence the production scale economics as large as the basis utilized in the present analysis. Cost-efficient HCOOH formation at a comparatively small scale for the chemical market can still be an emerging approach for ECR to be exercised commercially. Moreover, the carbon discharges credit concerning the sequestered CO2 for both systems had significant influence when optimistic assumptions were used, but it had a little influence under base-case assumptions. For ECR approach on the verge of financial competitiveness, a carbon emission policy can make a great difference for economic feasibility. Moreover, besides a power plant, CO2 could be attained from different alternative means such as cement industry, aluminium refining and fermentation process, and these alternative means emit CO2 in large concentrations, which can be utilized for ECR. Nevertheless, the above-discussed sensitivity analyses for both cases demonstrate that the impacts of the CO2 capture process in terms of operating and capital costs were low compared to the final LCF value. The improvement of techno-economic viewpoint for ECR to produce liquid fuels using the potential of alternative CO2 means is normally low.
It must be noted that ECR would only be beneficial in the context of CO2 mitigation if the energy operating the reduction process is carbon-neutral. Thermodynamically, it is suggested that the reduction of carbon dioxide requires a large amount of energy input compared to the energy obtained through the combustion forming the carbon dioxide. Undoubtedly, the overall EC cell power demand in both cases was higher compared to the 500 MW power plant producing carbon dioxide. The CO2 emissions will be increased if the electric power is obtained from traditionally consumed fossil fuels. Solar and wind-derived clear power means should be employed to operate the ECR, however, a big renewable energy facility will be required to thoroughly optimized the exhaust of a conventional power plant. Renewable electric power means supported by storage of clean energies will be needed to ascertain that the EC cell can operate at complete capacity. Although this will cause elevated electric power costs and subsequently high fuel expense compared to modeled base case in this study, the electric power costs didn’t show high sensitivity compared to the EC cell parameters.
7. Conclusions
We have presented the techno-economic feasibility of diesel fuel and formic acid produced through the electrochemical reduction of carbon dioxide obtained from the flue gases of a coal-derived power plant. The efficient ECR to carbon monoxide integrated with Fischer-Tropsch process to convert syngas into diesel fuel is found to be an attractive pathway towards a commercially feasible fuel. The ECR to produce formic acid was also studied, and it was seen that, as a bulk chemical, its price was almost the same as the market price. The EC cell high capital expenses were the main costs in each scenario, indicating that reduced EC cell cost concerning the electrode, high current density and improved faradaic efficiency are required to make both processes economically feasible.
Supporting Information
Supporting information such as techno-economic assumptions for capture and storage of CO2, electrochemical cell, CO2/CO separation and recycle, reference case techno-economic assumptions for Fischer-Tropsch system and other data related to this has also been provided.
Supporting Information is available at https://doi.org/10.33961/jecst.2021.00584
Acknowledgements
This work was supported by the China National Key Research and Development Plan Project (2018YFA0702300), National Natural Science Foundation of China (51950410590), Fundamental Research Funds for the Central Universities (HIT.NSRIF.2020054).