Investigation of Lithium Transference Number in PMMA Composite Polymer Electrolytes Using Monte Carlo (MC) Simulation and Recurrence Relation
Article information
Abstract
In this study, Monte Carlo (MC) simulation is conducted with recurrence relation to study the effect of SiO2 with different particle size and their roles in enhancing the ionic conductivity and lithium transference number of PMMA composite polymer electrolytes (CPEs). The MC simulated ionic conductivity is verified with the measurements from Electrochemical Impedance Spectroscopy (EIS). Then, the lithium transference number of CPEs is calculated using recurrence relation with the MC simulated current density and the reference transference number obtained. Incorporation of micron-size SiO2 (≤10 μm) fillers into the mixture improves the ionic conductivity from 8.60×10−5 S/cm to 2.35×10−4 S/cm. The improvement is also observed on the lithium transference number, where it increases from 0.088 to 0.3757. Furthermore, the addition of nano-sized SiO2 (≤12 nm) fillers further increases the ionic conductivity up towards 3.79×10−4 S/cm and lithium transference number of 0.4105. The large effective surface area of SiO2 fillers is responsible for the improvement in ionic conductivity and the transference number in PMMA composite polymer electrolytes.
1. Introduction
Solid Polymer Electrolytes (SPE’s) are electrolytes in solid form which consist of dissolved inorganic salts as charge carriers. SPEs eliminated the need for a separator. As a result, it is safer than traditional liquid and gel electrolytes. Besides, SPEs are extremely flexible and had better compatibility with most electrode materials. However, the application of SPEs on energy powered devices is hindered due to their high degree of crystallinity at room temperature. Due to the higher degree of crystallinity in traditional polymer electrolytes, i.e. PEO based polymer electrolytes, the motion on Li+ ions among the polymer chain is stalled, and this causes the film to generate a significant amount of internal resistance that results in low ionic conductivity and lithium transference number. Agrawal et al. [1] reported an ionic conductivity of 1×10−7 S/cm for PEO-LiClO4 at room temperature in their study of solid polymer electrolytes system. Also, Pozyczka et al. [2] reported a lithium transference number of 0.054 to 0.059 for PEO-LiTFSI SPEs with a molar ratio of 6:1 at 70°. Ionic conductivity and lithium transference number reported in the above studies for PEO SPEs are insufficient for practical application [3].
Moreover, ion transport in polymer electrolytes is a temperature-dependent process [4], ions received energy via thermal vibration to overcome the energy barrier so that they can have enough activation energy to hop to a nearby open vacant site. Thus, low activation energy and availability of open bond is essential for ionic transport in SPEs. Nevertheless, ionic conductivity and lithium transference numbers in SPEs can be boosted via the addition of ionic salts, plasticizers, and ceramic fillers [5–7].
Qian et al. [8] reported an improvement in ionic conductivity from 1.30×10−7 S/cm for (PEO)16LiClO4 to 2.36×10−4 S/cm at room temperature when 50 wt% Ethylene Carbonate (EC) was incorporated into the mixture. The addition of plasticizers lowers the glass transition temperature, Tg of the mixture, and this decreases the degree of crystallinity of polymer electrolytes at room temperature. As a result, the segmental motion of the polymer chain is enhanced, and the ionic conductivity increases. Xiao et al. [9] reported improvement in lithium transference numbers when ZrO2 particles were added into P(VDF-HFP)-PMMA matrix to create composite polymer electrolytes. They obtain lithium transference number of 0.28 to 0.41 for P(VDF-HFP)-PMMA-ZrO2 composite polymer electrolytes, an improvement from 0.16 without the addition of ZrO2 particles. The improvement in lithium transference number can be related to the large effective area of ZrO2 particles. The larger effective area provides Li+ ions more conduction pathways in the polymer matrix, this improves the dynamic of the polymer chain, thus yielding higher lithium transference number.
Although improving the performance of SPEs is an area of intense interest of many researchers, but there is not much attention paid in understanding the ionic transport mechanism in SPEs using particle simulation. Furthermore, the random behaviour of ions in the free volume also makes studying the transportation characteristics and conduction phenomenon of ions in polymer electrolytes challenging. Dynamic Bond Percolation (DBP) is one of the earliest models that describe ions motion in a statistically disordered and dynamically rearranging medium [10]. Not until Wagner et al. [11] introduced Monte Carlo (MC) model, a three-dimensional model for thermally activated ion transportation in a multi-well energy structure.
In this work, a stochastic Monte Carlo method using random sampling technique is employed to develop a model capable of study SPEs with minimal needs of experiment. PMMA is used as the host polymer and LiCF3SO3 as the dopant salt. The advantages of using LiCF3SO3 as the dopant salt are because of its resistance to oxidation, lower activation energy and, non-toxicity [7]. Besides, larger anion radius of LiCF3SO3 ensure a higher degree of dissociation of salt in the polymer matrix, this ensures better segmental mobility and yielding higher ionic conductivity [7,12]. Furthermore, PMMA as a semi-crystalline polymer is selected as the polymer host because of its higher ionic conductivity and lithium transference number at room temperature compared to traditional PEO based system that are suffering from low ionic conductivity and transference number at room temperature due to their high degree of crystallinity [13,14]. Several studies also show that PMMA based polymer electrolytes have the potential to be fabricated into solid electrolytes used in solid-state batteries and solid-state supercapacitors [15–17].
MC simulations are used to study the effect of SiO2 fillers with different filler sizes on the ionic conductivity, and the transference number of PMMA-LiCF3− SO3 system is calculated using recurrence relation. The simulation results are then compared to the measurement data to ensure the validity of the model.
2. Measurement and simulation of ionic conductivity and transference number
2.1 Sample preparation and characterization
The samples were prepared using solution cast method [14,18]. Poly(methyl-methacrylate) (PMMA), Ethylene Carbonate (EC), Lithium Triflate (LiCF3− SO3), and Silicon Dioxide (SiO2) in the experiment are obtained from Sigma Aldrich. PMMA (Mw – 996,000) were mixed the plasticizers, LiCF3SO3 and SiO2 inorganic fillers to yield a two-gram mixture. The mixture is first dissolved in Tetrahydrofuran (THF) solution and stirred using a magnetic stirrer until a homogenous solution is obtained. The homogenous solution was then poured into petri dishes and kept in a desiccator for drying until dry electrolyte films were obtained. Lastly, the films are cut into smaller samples, and the thickness of each sample was measured using micro-meter screw gauges and assembled into the battery cell for testing.
Gamry reference 600 series potentiostat was used for Electrochemical Impedance Spectroscopy (EIS) to characterize the polymer electrolytes samples. EIS is conducted in the frequency ranging from 0.1Hz to 1MHz at room temperature with the sample sandwiched between two spring-loaded stainless-steel electrodes, and the ionic conductivity is calculated by using the following equation:
where t (cm) is the thickness of SPE film, RB is the bulk resistance of electrolyte obtained from the Nyquist plot, and A (cm2) is the contact area between the film and the electrode, which equates to 2.56 cm2 in this work.
DC polarization measurement was also performed using Gamry reference 600 series potentiostat using a polarization voltage of 20mV to obtain the initial current, Io and steady-state current, Is. The transference number is calculated using Bruce-Vincent equation [19].
where ΔV is the polarization voltage, Ro and Rs are the resistance before and after polarization consisting of charge transfer resistance and passivating film resistance.
2.2 Monte Carlo Simulation
Interpreting the dynamics of ion in a dynamically disordered system like SPE is not an easy task as ions interact with each other in a polymer lattice, which is continually evolving with time. Nevertheless, by using MC simulation, it enables us to study the dynamic properties of ions by treating this complicated system as a grouping of many single ions. Hopping is the primary ion transport mechanism in a polymer. When an electric field was presence, Li+ ions start to drift; once the ions receive enough activation energy to overcome the energy barrier height and there was a free bond, the ions will hop to a nearby vacant site. In this study, the mass of Li+ ions was set to be 1.1525×10−26 kg and the electric field will be the moving force of the Li+ ions. The free-flight time and hopping events will be generated using a random number generator with a value of between 0 and 1. The wave vector,
where d represents the lattice spacing and p is the probability of observing an open bond at a nearby vacant site. The probability p is generated using a random number generator, 0 ≤ p ≤ 1, corresponding to the randomly appeared open bond in a polymer chain segment. The way of the ions transport in the polymer chain is known as “hopping.” Basically, ions that received enough energy to overcome the energy barrier height will hop to a nearby open bond, and it is a thermally activated process given by [4]:
, where (Ei – Ef) is the energy barrier height, vo is the escaping frequency of Li+ ions, T is the temperature in Kelvin, k represents the Boltzmann constant, Ei and Ef are the energy of the ions before and after hopping, respectively. The activation energy is set to be (< 0.2eV) as it was appropriate for SPEs [20]. The motion of Li+ ions is simulated to occur randomly within a time frame from t to t + 0.01 ps with their position updated every 0.01 ps. The wave vector obtained from the drifting process will be transferred to the hopping process for the verification of the hopping mechanism of Li+ ions and the state of Li+ ions after the hopping event. With all the parameters at hand, the drift velocity of Li+ v, mobility μ under the influence of electric field E using the following equation:
With mobility of ions obtained, the electric field dependent conductivity is calculated using:
, where n represents the concentration of ions, q is the total number of charged transferred by ions, and μ is the mobility of ions. The current density, J is then calculated using Ohm’s law:
3. Recurrence Relation
The lithium transference number is defined as the number of moles of lithium-ion transferred for one Faraday of charge transferred. In polymer electrolytes, the cation transference number is obtained by dividing the cation number over the number of ions transport across the cell. Current is the product due to transportation of Li+ ions. As such, the lithium transference number is determined using the ratio of steady-state current over the initial current in SPE.
In this work, the lithium transference number of PMMA SPEs is obtained using recurrence relation and the simulated current density. The transference number, t+si is derived based on recurrence relation developed by Matsuo et al. [21] and the steady-state current method introduced by Evans et al. [19]. Also, the initial and steady-state current densities in MC simulation are obtained through Ramo’s theorem [22].
The current density is given by i(t) = qv/d, where v represents the charge velocity, q is the amount of charge transferred, and d is the separation distance. The reference lithium transference number, t+si of PMMA-LiCF3SO3-EC is calculated as [19]:
, where Io and Iss(ref) is the measured initial and reference current densities for plasticized PMMA polymer electrolytes. Iss(ref) is used as reference current density for obtaining lithium transference number in PMMA-EC-LiCF3SO3-SiO2 using recurrence relation. The lithium transference number of PMMA-EC-LiCF3SO3-SiO2 is calculated as:
When more than one composition is incorporated into the mixture, this equation can be generalized using the recurrence relation, in which lithium transference number for various composition in polymer electrolytes is given as:
, where Issi is the steady-state current density in PMMA-LiCF3SO3-EC with different sizes of SiO2 fillers, Iss(ref) is the steady-state current density in PMMA-LiCF3SO3-EC, and t+ref is the lithium transference number of PMMA-LiCF3SO3-EC sample.
It is similar to the general expression of recurrence equation,
In this work, the ionic conductivity and steady-state current density of PMMA-LiCF3SO3-EC-SiO2 are simulated using MC simulation, and the lithium transference number is calculated using recurrence relation, based on the ratio of MC simulated steady-state current density by using lithium transference number of PMMA-LiCF3SO3-EC as reference. The results obtained are compared with experimental results and data from various works of literature.
3. Results and Discussion
3.1 Ionic Conductivity of SPEs
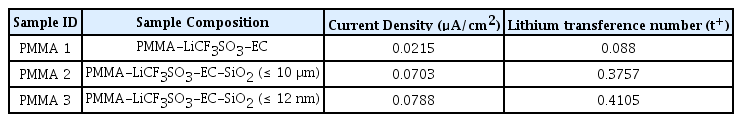
Fig. 1 shows the Nyquist of PMMA-LiCF3SO3-EC SPE film at 27°C. The bulk resistance, Rb is determined from the Nyquist plot with a value of 97.93Ω. Experimental measurements for PMMA-LiCF3SO3-EC, PMMA-LiCF3SO3-EC-SiO2 (≤10μm), and PMMA-LiCF3SO3-EC-SiO2 (≤12nm) is tabulated in Table 1. The ionic conductivity obtained via MC simulation is shown in Fig. 2, and results from MC simulation agree well with experimentally obtained data.
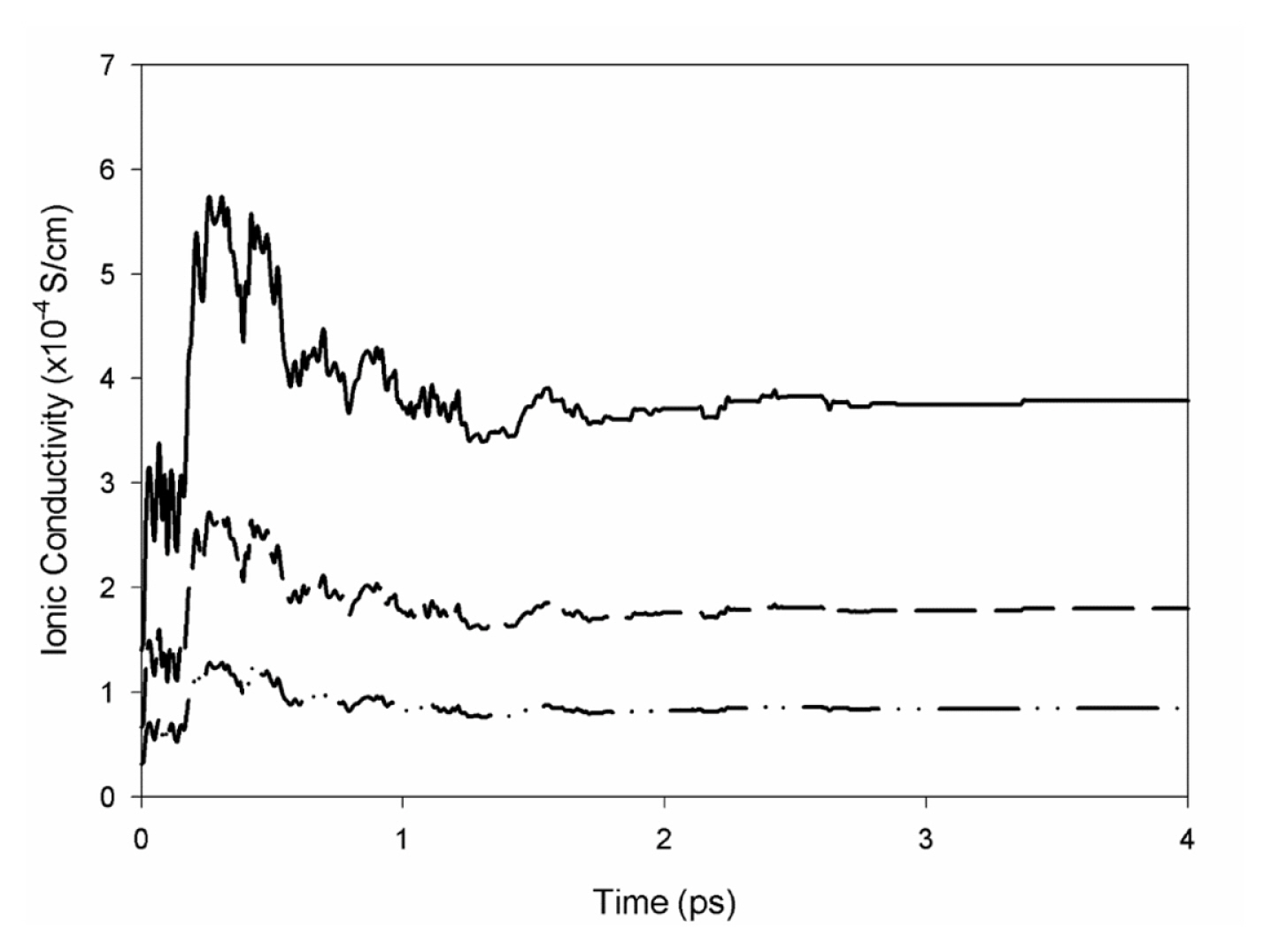
Monte Carlo ionic conductivity simulation of PMMA 1 (dash-dot line), PMMA 2 (dash line), and PMMA 3 (solid line) SPE samples.
It is shown that PMMA1 sample which does not contain ceramic filler has the lowest conductivity of 8.60×10−5 S/cm, a value comparable to work from Pal et al. [23], where they reported a conductivity of 3.52×10−5 S/cm for plasticized PMMA-LiClO4. The low ionic conductivity is mainly contributed by the crystallinity nature of PMMA at room temperature, where Li+ ions are believed to suffering from low mobility and are trapped within the polymer chain.
With the addition of SiO2 (≤10μm), ionic conductivity of 2.35× 10−4 S/cm is achieved for PMMA2 sample. Chew et al. [24] also reported an improvement in ionic conductivity when they added micron size SiO2 particles into PMMA based polymer electrolytes. They reported an ionic conductivity of 2.15×10−4 S/cm for PMMA-LiCF3SO3-EC-SiO2, an improvement from 1.36×10−5 S/cm without SiO2 fillers in their study.
In this study, nano-sized SiO2 filler is also added into the polymer matrix. The addition of nano-sized filler further increases the ionic conductivity up to 3.79×10−4 S/cm for PMMA3 samples. The improvement in ionic conductivity agrees well with [25,26] findings where conductivity enhancement increases with increasing effective surface area of filler grains, as nano-sized SiO2 has a greater surface area than micron-sized SiO2 fillers. The improvement in ionic conductivity is associated with the increase in conduction pathway benefitted from the larger effective surface area of SiO2 particles.
Moreover, the addition of SiO2 fillers disrupts the crystal structure of PMMA matrix. As a result, it lowers the ionic attraction force and improves Li+ ions chain mobility. Dissanayake et al. [25] studied the effect of particle size on the ionic conductivity of PEO based polymer electrolytes. They reported the conductivity was significantly improved with the smallest filler size having the larger effective surface area. By decreasing the particle size from 10μm to 10nm, ionic conductivity was increased by one order of magnitude at 70°C.
Furthermore, the improvement in ions mobility is also verified through MC simulation. Results from simulation show an increase in the number of ions travelling through the polymer, from 8.50×1010 for PMMA1 samples up to 3.80×1011 for PMMA3 samples. The increasing number of ions travelling through the polymer is directly related to the mobility improvement of Li+ ions, since there are more space and larger free volume in the polymer matrix for Li+ ions to travel around more easily [25,27].
3.2 Transference number calculation using recurrence relation
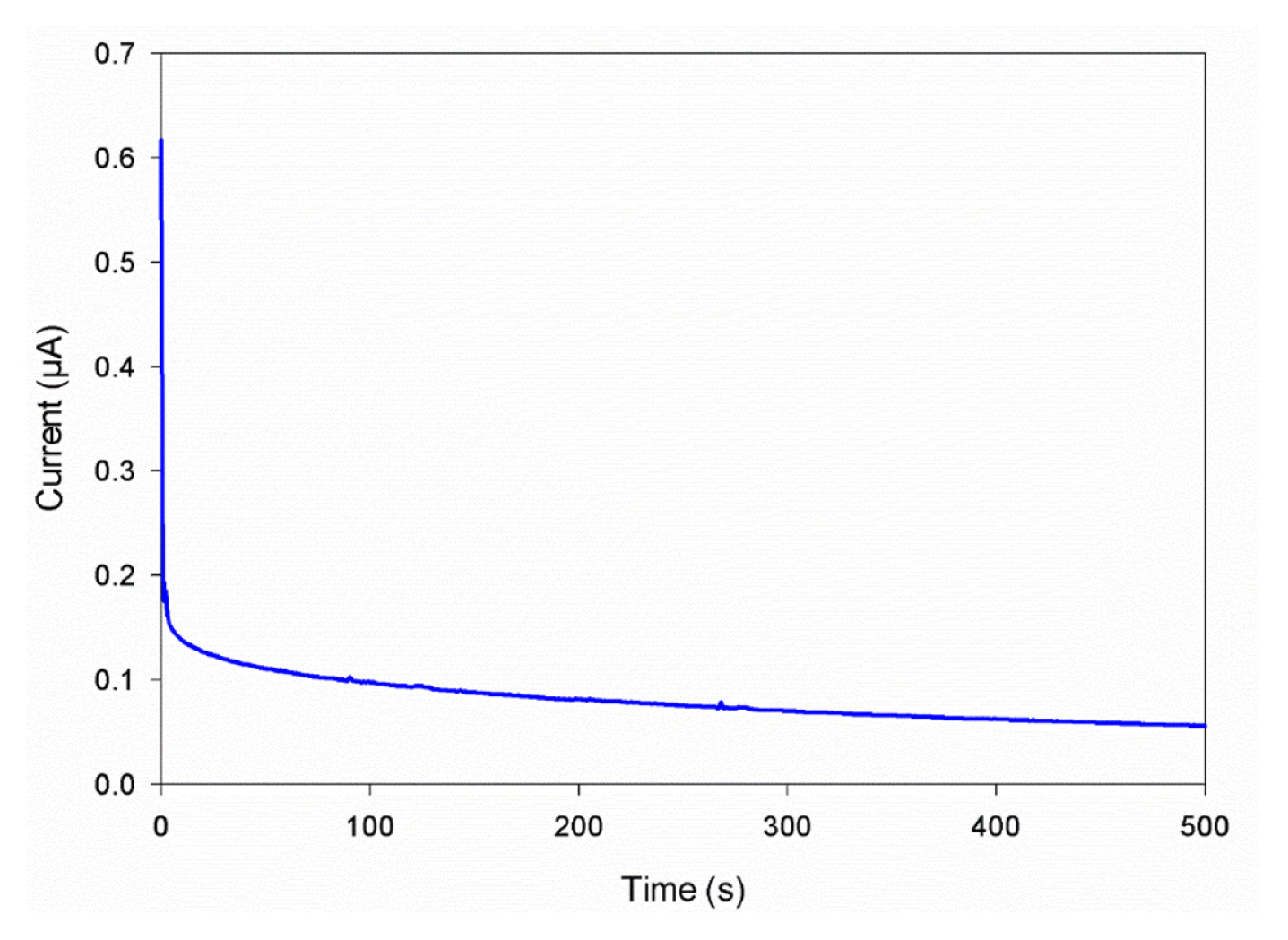
Fig. 3 shows the dc polarization curve for PMMA1 samples, the lithium transference number is calculated using Bruce-Vincent method developed by Bruce et al. [19], and it is found to be 0.088. The obtained lithium transference number for PMMA1 samples is used as a reference for the calculation of lithium transference number for PMMA2 and PMMA3 samples by using recurrence relation and MC simulation obtained current density. Fig. 4 compares the experimentally obtained steady-state current density with MC simulated current density under an electric field of 10 V/m for PMMA1, PMMA2, and PMMA3 samples, respectively.

Steady-state current density of PMMA SPEs with and without SiO2 fillers (dashed line: MC simulation, dotted: Measurements).
It is known that cations are responsible for the generation of electricity inside the battery, and the current density produced is dependent on the ionic conductivity of SPE inside the battery. As a result, PMMA1 samples that have the lowest ionic conductivity produces the lowest current density of 0.0214 μA/cm2, and the ionic conductivity increases up to 0.0703 μA/cm2 for PMMA 2 samples with the addition of SiO2 (≤10 μm). The highest current density of 0.0758 μA/cm2 is obtained for PMMA3 samples that exhibit the maximum ionic conductivity. With the current density obtained from all samples and lithium transference number for PMMA1 samples, the lithium transference number for PMMA2 and PMMA3 samples are calculated using recurrence relation. Table 2 shows the transference number of PMMA-LiCF3SO3-EC solid polymer electrolytes with and without SiO2 fillers.
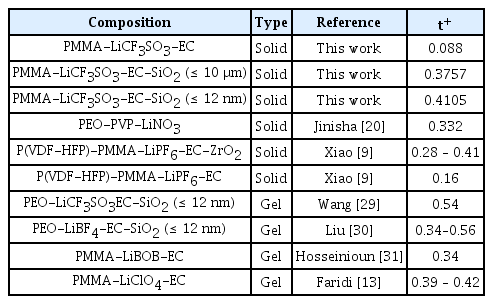
Table 3 shows the lithium transference obtained in this work compared to the lithium transference number obtain from other literature, and it is shown that the lithium transference number obtained using recurrence relation is reasonable. The highest lithium transference number obtained in this study is 0.4105 for PMMA-LiCF3SO3-EC-SiO2 (≤ 12 nm), which is comparable to lithium transference number reported by Xiao et al. [9] in their study of P(VDF-HFP)-PMMA hybrid polymer electrolytes doped with ZrO2 particles. Furthermore, Liu et al. [28] reported a maximum lithium transference number of 0.39 for PEO CPEs doped with Ga-LLZO nanofillers. Wang et al. [29] and Liu et al. [30] obtained lithium transference number of up to 0.54 and 0.56 respectively in their study of PEO gel polymer electrolytes (GPEs) doped with SiO2 nano-particles. Naturally, lithium transference number obtained for GPEs would be higher than that of SPEs, because GPEs are more amorphous than SPEs, and the mobility of Li+ ions in GPEs is higher, yielding a higher lithium transference number.
4. Conclusion
A sophisticated MC model had been developed in this study, the model is capable of simulating the ionic conductivity of PMMA-LiCF3SO3-EC of various compositions at room temperature, and the simulation data agree well with experimental results. The results also illustrate that PMMA CPEs with SiO2 particles greatly enhance the ionic conductivity and lithium transference number in comparison with the traditional SPEs at room temperature. Since cations are the primary charge carriers in the system, the enhancement in ionic conductivity produces higher current density and larger cationic transference number. The highest ionic conductivity and current density achieved at room temperature in this work are 3.79×10−4 S/cm and 0.0788 μA/cm2, respectively, for PMMA-LiCF3SO3-EC-SiO2 (≤ 12 nm). The highest lithium transference number recorded is 0.4105 with 3.8×1011 cm−3 Li+ ions travelling through the PMMA polymer electrolyte for nano-sized SiO2 particles in the simulation. Lastly, it is shown that the addition of inorganic particle into the polymer matrix greatly enhances the ionic conductivity and lithium transference number of traditional electrolytes system. CPEs not only has a greater ionic conductivity and lithium transference number than traditional polymer electrolytes system, but several recent studies also show that CPEs exhibit better thermal stability and energy density [16,32].
Acknowledgement
This work is supported by FRGS/2019, Ministry of Higher Education, Malaysia.
Notes
Declaring of Competing Interest
I hereby declare that I have no competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.