Electrochemical Properties of Acetylene Black/Multi-walled Carbon Nanotube Cathodes for Lithium Thionyl Chloride Batteries at High Discharge Currents
Article information
Abstract
Lithium thionyl chloride (Li/SOCl2) batteries exhibit the highest energy densities seen in commercially available primary batteries because of their high operating voltages and discharge capacities. They are widely used in various extreme environments; however, they show signs of degradation at high discharge currents. The discharge performance of Li/SOCl2 is considered to be greatly dependent on the carbon materials used in the cathode. Therefore, suitable carbon materials must be chosen to improve discharge performances. In this work, we investigated the discharge properties of Li/SOCl2 batteries in which the cathodes contained various ratios of acetylene black (AB) and multi-walled carbon nanotubes (MWCNTs) at high discharge currents. It was confirmed that the MWCNTs were effectively dispersed in the mixed AB/MWCNT cathodes. Moreover, the discharge capacity and operating voltage improved at high discharge currents in these mixed cathodes when compared with pure AB cathodes. It was found that the mesopores present in the cathodes have a strong impact on the discharge capacity, while the macropores present on the cathode surface influence the discharge properties at high discharge rates in Li/SOCl2 batteries. These results indicate that the ratio of mesopores and macropores in the cathode is key to improving the discharge performance of Li/SOCl2 batteries, as is the dispersion of the MWCNTs.
1. Introduction
Lithium thionyl chloride (Li/SOCl2) primary batteries have been widely used as power sources in extreme environments because of their high operating voltage (3.6 V), wide operating temperature range (−55–85°C), and high discharge capacity (0.8 Ah, AA size, wound type) [1–4]. In general, Li/SOCl2 batteries consist of a lithium metal anode, a carbon-based cathode, and SOCl2 containing a lithium salt as the electrolyte solution. During the discharge process, the lithium metal is oxidized and the SOCl2 electrolyte solution is reduced to produce sulfur, sulfur dioxide, and lithium chloride. The carbon cathode acts as a catalytic surface for the reduction of the SOCl2 [1–3], and the surface structure of the carbon cathode is of particular importance due to the formation of insoluble reaction products such as lithium chloride on the carbon surface [5–11]. Lithium chloride exhibits a low electrical conductivity, thereby hindering the reduction of SOCl2, and so the presence of lithium chloride in the pores of the carbon cathode can make the diffusion of SOCl2 during the discharge process more difficult. These issues must therefore be addressed to allow for the broader application of Li/SOCl2 batteries.
The discharge performances of Li/SOCl2 batteries are greatly dependent on the physicochemical properties of the carbon cathode materials employed. For example, carbon nanotubes (CNTs) could improve the discharge performance at high discharge currents due to their high electrical conductivity and high specific surface area. As such, the catalytic effects of CNTs in Li/SOCl2 batteries have been widely investigated [12–19]. However, the impact of their structure on the discharge performance of Li/SOCl2 batteries is not yet clearly understood. Additionally, their nanostructures allow the facile formation of aggregates, which may cause a deterioration in the battery performance. Therefore, technologies aimed at the dispersion of CNTs in the cathodes are key to improving the discharge performances of Li/SOCl2 batteries. Commercially, mixtures of acetylene black (AB) and ketjen black have been widely used as a cathode material in Li/SOCl2 batteries. However, the discharge performances of such systems at high discharge rates are generally poor under extreme environments. To address this issue, we wished to consider a new composite electrode, namely AB/multi-walled carbon nanotube (MWCNT), as the battery cathode material, with the aims of improving the discharge performance at high discharge rates. Thus, we herein report our investigation into the effect of AB addition on the effective dispersion of MWCNTs in cathodes, and the impact of mixing AB and MWCNTs on the discharge performance of Li/SOCl2 batteries by investigating the discharge properties of AB/MWCNT mixed cathodes at discharge currents of 150 and 600 mA. In addition, the correlation between the discharge properties and the physical properties of AB/MWCNT mixed cathodes are discussed.
2. Experimental
2.1 Preparation of cathode materials
Cathode materials were prepared using AB (Denka black, Denka Co. Ltd., Tokyo, Japan) and MWCNTs (LUCAN BT1003M, LG Chem. Seoul, Korea) with AB:MWCNT weight ratios of 0:10, 3:7, 5:5, 7:3, and 10:0. Isopropyl alcohol (99.7%, Sigma-Aldrich Co., St. Louis, USA) was added to the cathode materials and these were stirred for 10 min. Subsequently, pastes were prepared by mixing the cathode materials and polytetrafluoroethylene (POLYFLON PTFE D-Series, DAIKIN Industries Ltd., Osaka, Japan) in a 10:1 weight ratio and stirring for 5 min. Expanded nickel mesh (VITZRO CELL Co. Ltd. Chungnam, Korea) was used as a current collector, and the pastes were pressed onto the mesh by roll-pressing. The cathode materials were loaded on the current collector at a loading of ~0.6 g. To maintain a constant mass loading, the thickness of the cathode was fixed at 0.7 mm, and the cathodes were dried at 120°C for 4 h under vacuum.
2.2 Materials characterization
Scanning electron microscopy (SEM) was carried out to investigate the morphologies of the AB/MWCNT mixed cathodes (S-4800, Hitachi Ltd., Tokyo, Japan). The specific surface area and pore size distribution were investigated by nitrogen adsorption-desorption isotherm measurements (ASAP 2420, Micromeritics Instrument Corp., Norcross, USA).
2.3 Electrochemical measurements
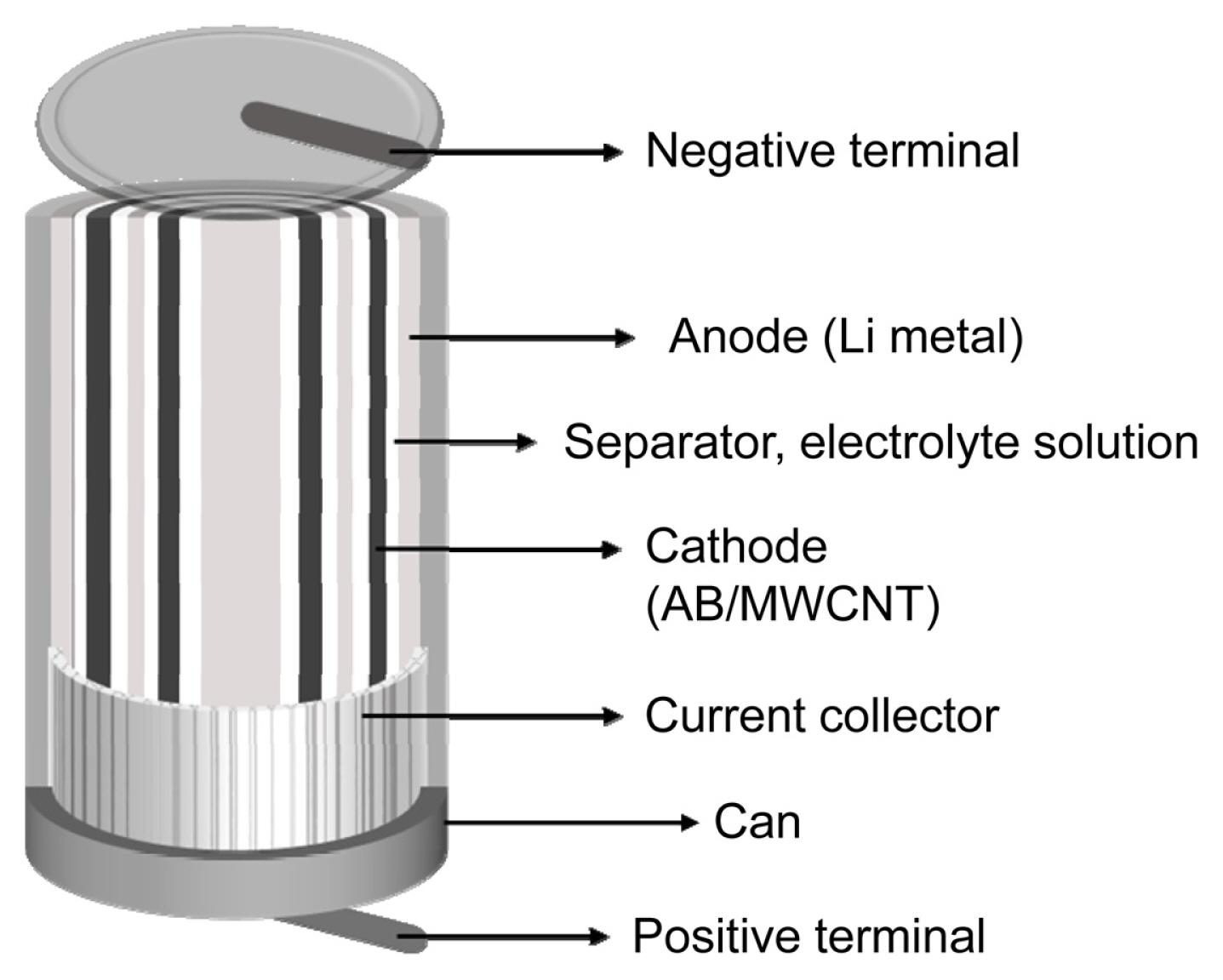
Electrochemical tests were performed using commercial wound-type batteries (AA size), as shown in Fig. 1. Lithium metal (VITZRO CELL Co. Ltd. Chungnam, Korea) was used as the anode and the AB/MWCNT mixed materials were used as the cathode. In addition, 1.25 M LiAlCl4 (98%, BASF SE, Ludwigshafen, Germany)/SOCl2 (99.6%, LANXESS, Cologne, Germany) was selected as the electrolyte solution. The discharge tests were performed between the open circuit voltage (OCV) and 2.0 V at both 150 and 600 mA (Series 3600, MACCOR Inc., Oklahoma, USA). Nyquist plots of the Li/SOCl2 batteries were obtained before discharge testing at OCV by electrochemical impedance spectroscopy (ZIVE MP5, WanATech Co. Ltd., Seoul, Korea). The frequency range was 100 kHz–10 mHz with an alternating amplitude of 10 mV.
3. Results and Discussion
3.1 Physical properties of the AB/MWCNT cathodes
Fig. 2 shows SEM images of the cathodes prepared using various AB:MWCNT ratios. In the pure MWCNT cathode, mostly aggregated linear structures were observed, while spherical structures were confirmed in the pure AB cathode (Figs. 2a and 2b). In the case of AB:MWCNT (7:3), AB was dominant on the cathode surface (Fig. 2c), while increasing amounts of MWCNTs were observed on the cathode surface as the MWCNT content was increased (Figs. 2d and 2e). Moreover, MWCNT aggregates were not confirmed in AB/MWCNT mixed cathodes, indicating that MWCNTs were effectively dispersed by the addition of AB. These results revealed that the morphology of carbon cathodes is largely dependent on the ratio of AB to MWCNTs.

SEM images of the AB and MWCNT mixed cathodes, with AB:MWCNT ratios of (a) 0:10, (b) 10:0, (c) 5:5, (d) 7:3, and (e) 3:7.
The adsorption and desorption isotherms of the AB/MWCNT mixed cathodes are shown in Fig. 3. The isotherms are type IV, and the hysteresis indicates the existence of mesoporous structures within the cathodes (Fig. 3a) [14]. The largest specific surface area was observed for the pure MWCNT electrode, which was attributed to the smaller diameters of the MWCNTs, as well as their linear shape compared to that of AB. The average diameter and the average length of the MWCNTs were estimated to be 13 nm and 12 μm, respectively, and the average diameter of AB was estimated to be 36 nm. The calculated values (Fig. 3b) were obtained by interpolation between the values of MWCNTs and AB. It was confirmed that the measured specific surface areas of AB/MWCNT mixed cathodes were larger than the calculated values, indicating that the cavities between the MWCNT particles were filled with AB particles. Indeed, large numbers of cavities were observed in the MWCNT aggregates, as shown in Fig. 2(a). Fig. 3(c) shows the pore size distributions of the AB/MWCNT mixed cathodes, and detailed specific surface areas and specific pore volumes are shown in Table 1. Micropores with diameters of less than 2 nm were negligible in all electrodes, making up only 0.1%, while mesopores with diameters of 2–50 nm were dominant (>63.5%), with a low amount of macropores being detected in all cases, although this increased upon increasing the AB content. This result indicates that the ratio of AB to MWCNTs in mixed cathodes has a significant impact on the porosity of the system, which might be expected to alter the discharge properties of Li/SOCl2 batteries constructed using these electrodes.
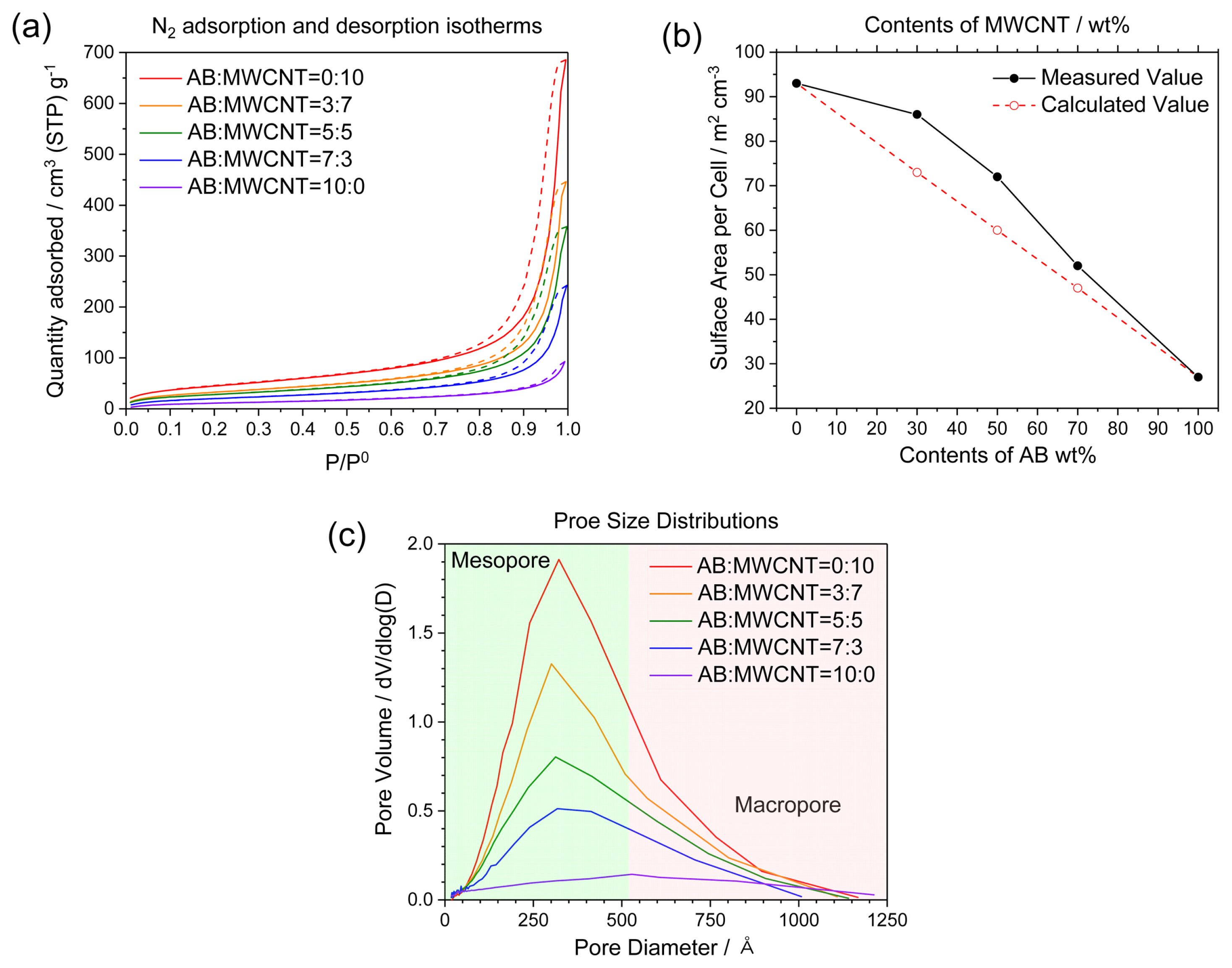
Nitrogen adsorption/desorption isotherms and pore size distributions of the AB and MWCNT mixed cathodes.
3.2 Electrochemical properties of the AB/MWCNT cathodes in Li/SOCl2 batteries
Fig. 4 shows the discharge properties of the Li/SOCl2 batteries prepared using cathodes containing various AB:MWCNT ratios at 150 and 600 mA. At a discharge current of 150 mA, both the discharge time and the operating voltage increased in the MWCNT-containing cathodes compared with the pure AB cathode (Fig. 4a). For the AB/MWCNT mixed cathodes, the specific surface area increased upon increasing the MWCNT content (see above), which provided larger reaction sites for the formation of LiCl. This result indicates that the discharge capacities of these Li/SOCl2 batteries at relatively low discharge currents are affected by the specific surface area. However, different discharge properties were confirmed for the AB/MWCNT mixed cathodes at a discharge current of 600 mA (Fig. 4b). More specifically, discharge times were longer for the cathodes containing MWCNTs compared to those observed for the pure AB cathode. In addition, the discharge times gradually increased upon increasing the AB content when using the mixed AB/MWCNT cathodes. This result implied that the discharge performance at a high discharge current was enhanced for MWCNT cathodes upon the addition of AB. In MWCNT cathodes without AB, the majority of the MWCNTs were aggregated, leading to a deterioration in the discharge performance of Li/SOCl2 at a high discharge current of 600 mA. In addition, LiCl forms more rapidly on the cathode surface at high discharge currents, causing pore closure in a significant number of mesopores. The longest discharge time was observed when using a mixed AB/MWCNT cathode with a ratio of 7:3. In this case, AB was predominant on the surface, leading to a greater number of macropores than in the case of the AB/MWCNT cathodes with ratios of 5:5 and 3:7 (Fig. 3 and Table 1). It was therefore apparent that the increased number of macropores on the cathode surface improved the discharge performance at high discharge rates. However, the AB cathode exhibited the lowest discharge capacity despite having the highest ratio of macropores on its surface, indicating that macropores alone do not determine the discharge capacity of Li/SOCl2. Instead, they assist the diffusion of SOCl2 toward the carbon cathode during the discharge process. It can therefore be considered that the discharge properties at a high discharge current of 600 mA are dependent on the number of macropores on the cathode surface, as well as the dispersion of MWCNTs.
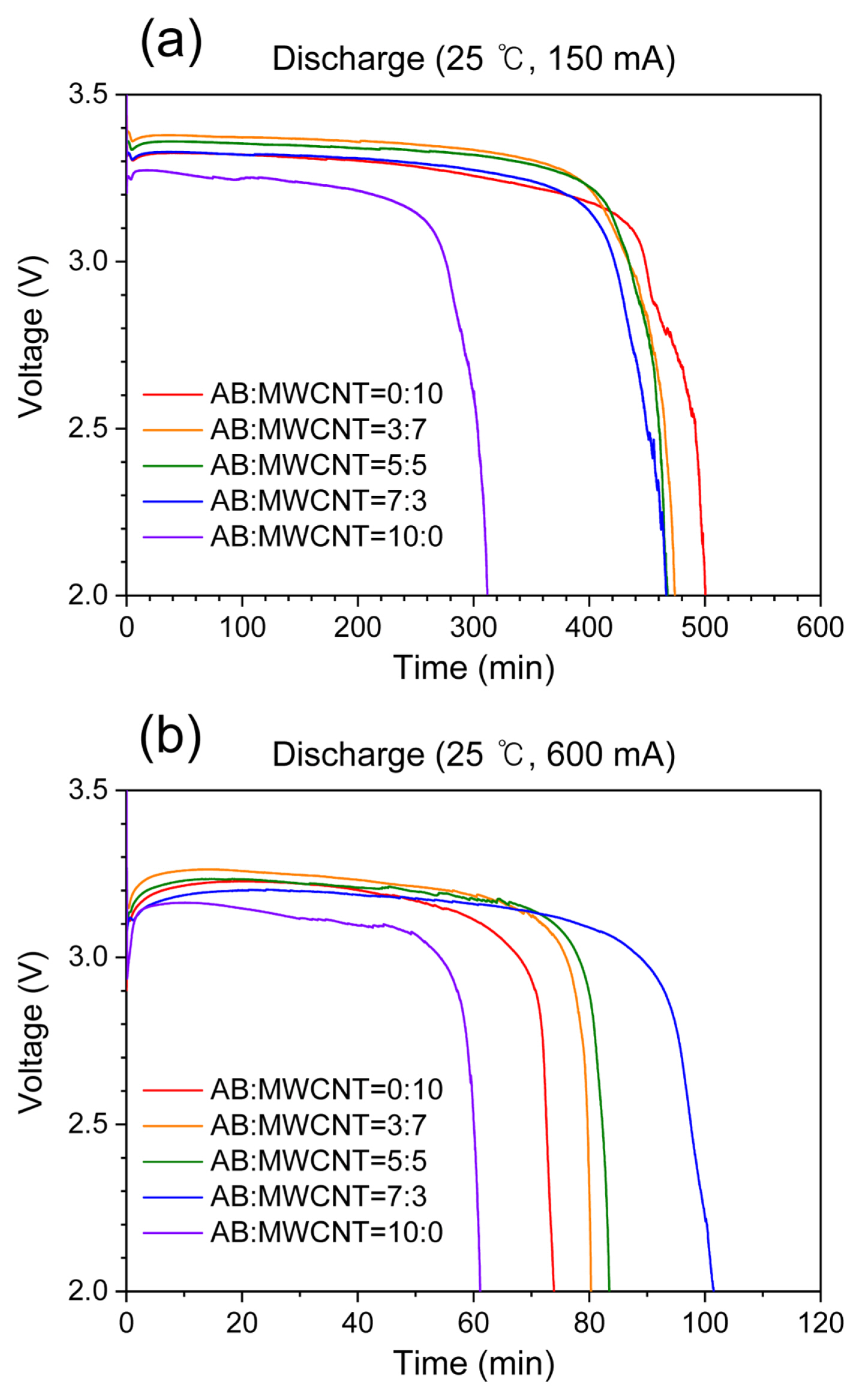
Discharge curves of Li/SOCl2 batteries prepared using cathodes containing various AB:MWCNT ratios at discharge currents of (a) 150 and (b) 600 mA.
Therefore, the ratio of mesopores to macropores in the cathode is important in the context of attempting to improve the discharge performance of Li/SOCl2 at high discharge currents. Since the surface resistance of the carbon cathode is considered to have an influence on the discharge properties of Li/SOCl2 batteries at high discharge currents, electrochemical impedance spectroscopy was carried out on the AB/MWCNT cathodes. The equivalent circuit and Nyquist plots of Li/SOCl2 batteries with cathodes containing different ratios of AB/MWCNT before discharge are shown in Fig. 5. The Nyquist plots shows two semicircles and one slope, whereby Rs, R1, R2, C1, C2, and Zw (Fig. 5a) represent the solution resistance, surface film resistance, charge transfer resistance, surface film capacitance, double layer capacitance, and diffusion resistance, respectively [20–22]. The surface film resistance of the pure MWCNT cathode in the high frequency region was significantly larger than those seen for the mixed AB/MWCNT cathodes. This was a result of the high percentage of mesopores in the pure MWCNT cathode, as well as increased MWCNT aggregation. In addition, the surface film resistance decreased as the ratio of MWCNTs in the mixed AB/MWCNT cathodes decreased. Based on the above SEM images and pore size distributions, it appeared that an increased AB content resulted in the generation of numerous macropores on the cathode surface. Moreover, the MWCNTs are more effectively dispersed in the mixed AB/MWCNT cathodes, and so the surface film resistance in the cathode was reduced upon the addition of AB to the MWCNTs. The slopes shown in the figure correspond to the Warburg impedance element, and are attributed to diffusion resistance in the cathodes. The diffusion coefficients were calculated using the following equation [23,24]:
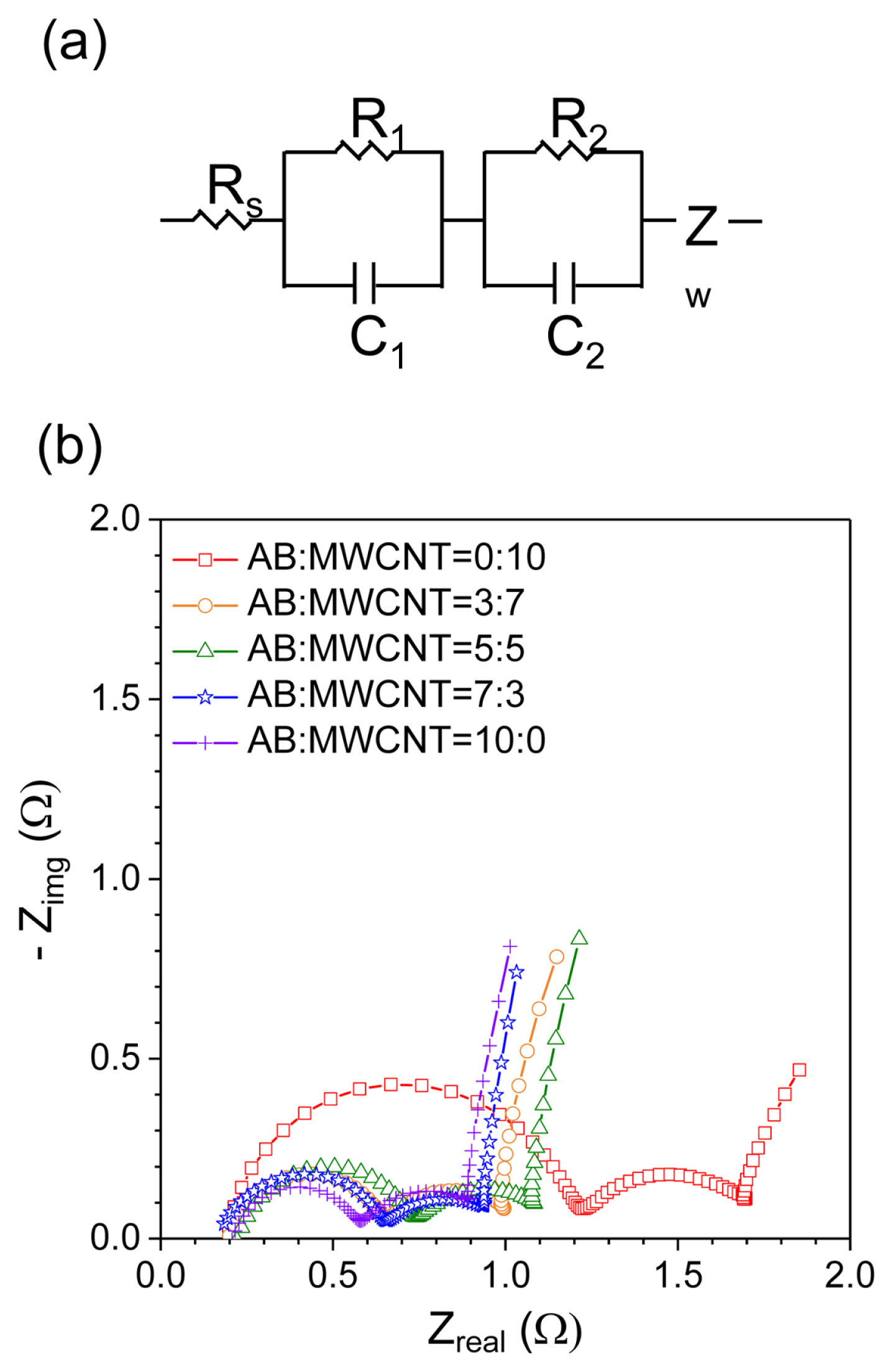
(a) Equivalent circuit, and (b) Nyquist plots prior to discharge for the Li/SOCl2 batteries in which the cathodes contained various AB:MWCNT ratios.
where D, R, T, A, n, F, C, and σw represent the diffusion coefficient, gas coefficient, absolute temperature, electrode area, number of electrons per molecule, Faraday’s constant, molar concentration of lithium ions, and Warburg coefficient, respectively. The diffusion coefficients were estimated to be 7.10 × 10−10, 1.74 × 10−9, 2.08 × 10−9, 4.00 × 10−9, and 2.45 × 10−9 cm2 s−1 for AB:MWCNT = 0:10, 3:7, 5:5, 7:3, and 10:0, respectively. In the mixed AB/MWCNT cathodes, the diffusion coefficient increased upon decreasing the MWCNT content, which indicates that diffusion was faster in the mixed cathodes than in the pure MWCNT cathode. This result supports the discharge properties observed at a high discharge current of 600 mA.
Based on these results, it therefore appears that the surface condition of carbon cathodes has an influence on the discharge performance of Li/SOCl2 at high discharge currents. However, it alone does not determine the discharge capacity, as indicated by the short discharge time in the AB cathode. These results suggest that surface conditions should be controlled to give a relatively high macropore content to improve the rate performance. Moreover, the discharge capacity is determined by the mesopore ratio in the bulk of the carbon cathode.
4. Conclusions
We herein reported our investigation into the discharge properties of Li/SOCl2 batteries in which the cathodes contained various ratios of AB and MWCNTs. For the purpose of this study, discharge currents of 150 and 600 mA were employed. SEM observations confirmed that the morphologies were significantly different in mixed AB/MWCNT cathodes. In addition, in the pure MWCNT cathodes, the majority of MWCNTs were aggregated, while they were effectively dispersed in mixed the AB/MWCNT cathodes, which resulted in a decrease in the surface resistance. Furthermore, pore size distribution analysis showed an increase in the mesopore ratio in the cathodes as the MWCNT content was increased. Moreover, the discharge times of Li/SOCl2 batteries increased upon increasing the MWCNT content in mixed AB/MWCNT cathodes at a discharge current of 150 mA due to the greater specific surface area and larger number of mesopores. However, the discharge time decreased upon increasing the MWCNT content in the mixed AB/MWCNT cathodes at a discharge current of 600 mA. In this case, AB was dominant on the surface of the cathodes, leading to the formation of numerous macropores on the surface. This result indicates that the discharge capacity is affected by the ratio of mesopores in the carbon cathodes. The macropores present on the surfaces of the carbon cathodes also influence the discharge performance of Li/SOCl2 at high discharge currents. Thus, from our results, it is apparent that the discharge performance of Li/SOCl2 batteries can be improved by using mixed AB/MWCNT cathodes. In future work, the interfacial reactions that occur between the SOCl2 electrolyte and the cathode during the discharge process should be clarified to allow for commercialization.
Acknowledgement
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20184030202130). This work also received support from the Soonchunhyang University Research Fund.