1. Introduction
Magnesium alloy is very prospective for applications in many fields such as aerospace, electronics, medical, automobile and military manufacturing due to its excellent specific strength, thermal conductivity, processing performance and relatively low cost [1–10]. However, one of the main reasons limiting the application of magnesium alloy is its poor corrosion resistance in the aqueous environment. The oxide film produced by the oxidation of magnesium is porous, which cannot effectively prevent the corrosion of magnesium matrix [11,12]. Besides, because of the low corrosion potential of pure magnesium (the standard electrode potential is only −2.73V), other metallic elements in the alloy, which have higher corrosion potential than magnesium, are easy to form galvanic corrosion with magnesium to accelerate corrosion process of the alloy [13–15]. The ability to form galvanic corrosion between different metals and magnesium is different but there are few reports about the galvanic corrosion between different alloying elements and magnesium [16,17]. The cathodic reaction for Mg alloys in aqueous solution is mainly hydrogen evolution reaction. Thus, measuring the hydrogen evolution ability can indirectly evaluate the extent of galvanic corrosion between magnesium and other metals. Therefore, this work studied the cathodic hydrogen evolution ability of different pure metals which either can be added in Mg as alloying elements or introduced as impurities. Further, the long term galvanic corrosion behavior between Mg and other metals was also investigated to clarify the change of the hydrogen evolution ability of different metals with corrosion progressing due to film formation.
2. Experimental Procedures
The cathodic electrodes used for this investigation were pure metals (Ti, Zn, Cu, Fe, W, Cr, Co, Al, Ag, Sn and Zr). They are selected since these elements are the common alloying elements or impurities. The surface area of each used cathodic electrode is 0.5 cm×0.5 cm. The purity of all studied metals is higher than 99.9 wt% and the purity of pure Al and Sn is higher than 99.99 wt%. All electrochemical experiments were carried out in 0.6 M NaCl solution. The electrodes were uniformly polished using 400#, 800#, 1200#, 1500# and finally 2000# grit SiC paper. The electrochemical test device used in experiments is Versa STAT V3 electrochemical workstation produced by US Princeton Corporation. A three-electrode system is applied, with a saturated calomel electrode (SCE) as the reference electrode, a platinum plate as the counter electrode, and samples as the working electrode. The scan rate was 5 mV/s for cathodic polarization. Using this relatively fast scan rate is to minimize the variation of surface condition during cathodic polarization. The scan rate was 0.5 mV/s for anodic polarization measurement. Using this relatively slow scan rate is to allow the formation of oxide film and obtain accurate breakdown potential of the film. A big piece of pure Mg, with exposed surface area of 6.0 cm×12.0 cm, was used as anode and pure metal electrodes were used as cathode in galvanic corrosion test. The surface area of pure Mg (anode) is much bigger than that of other pure metals (cathodes). This is to ensure the mixed corrosion potential is close to Mg, which is similar to the condition of Mg alloys. Before galvanic corrosion, the pure Mg was soaked into 0.6 M NaCl solution for about 30 min until the corrosion potential reached relatively stable, and then other pure metal was connected to start the galvanic corrosion test. The galvanic corrosion test was performed by the electrochemical workstation mentioned above.
3. Results and Discussion
3.1 Cathodic polarization tests
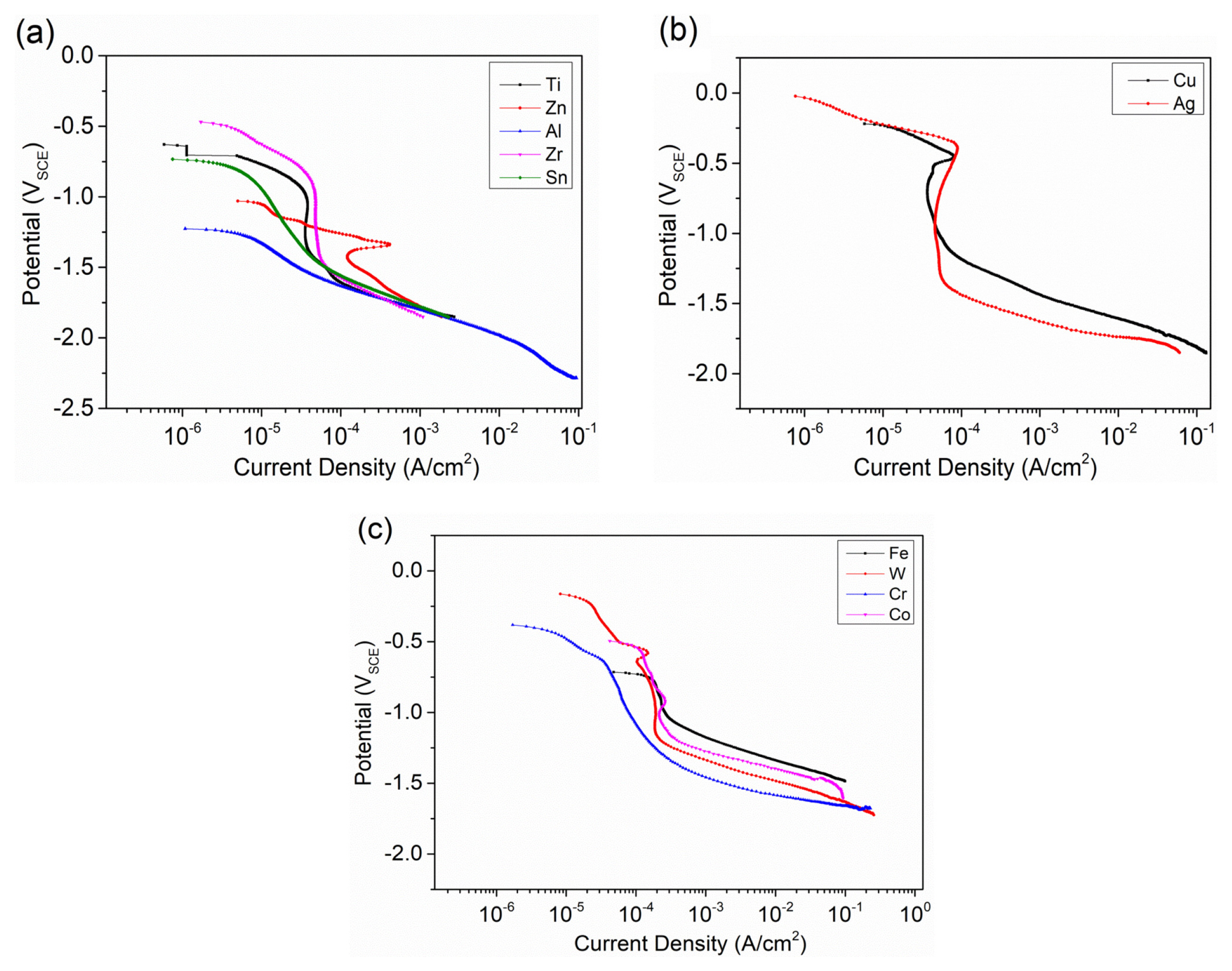
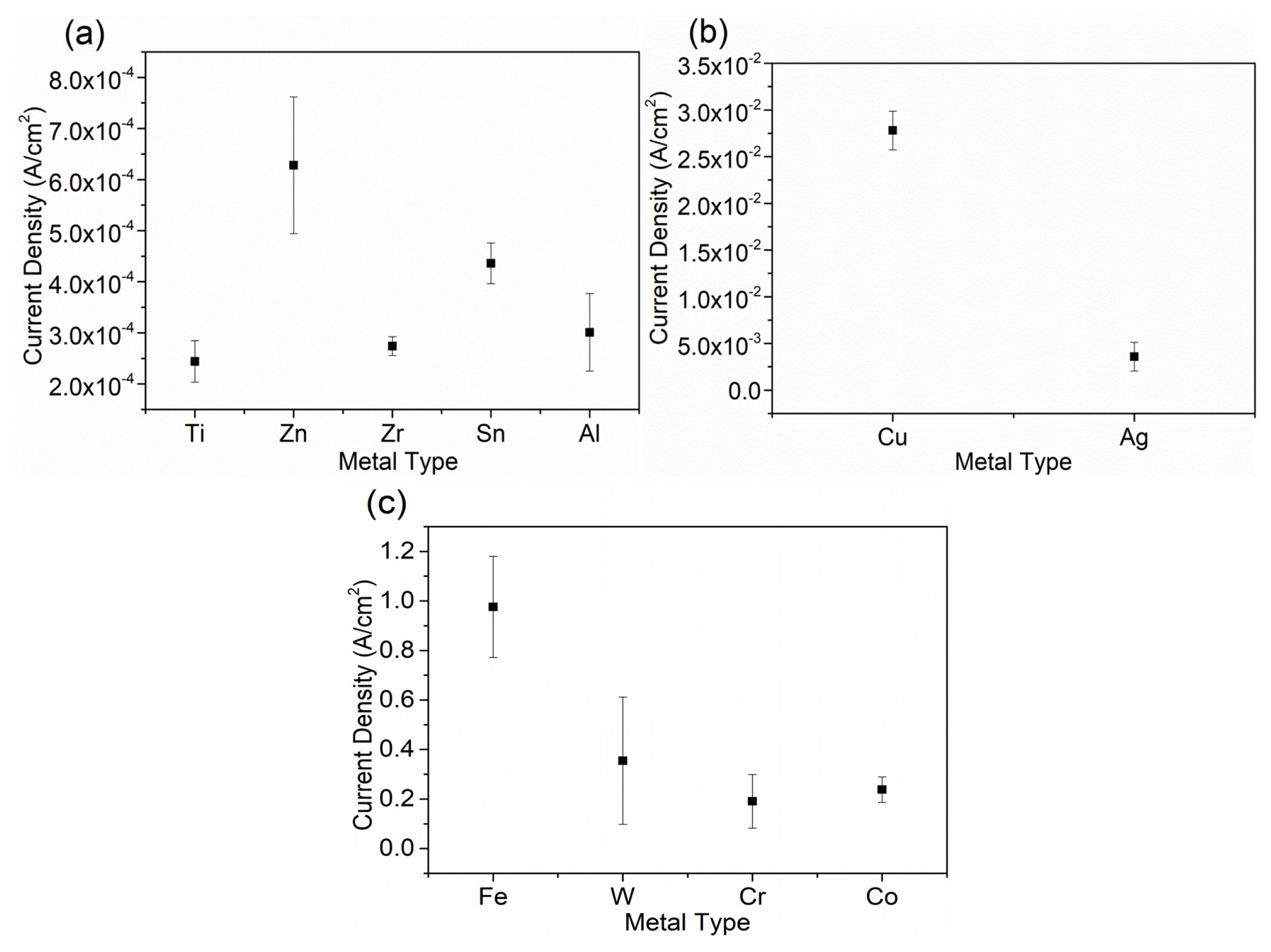
After obtaining the data of cathodic polarization tests on the pure metals, the ohmic potential drop (IR Drop) was removed, and the corresponding cathodic polarization curves were shown in Fig. 1. Since the corrosion potential of Mg alloys is about −1.7 VSCE, the cathodic current density for different metals at −1.7 VSCE was plotted and compared, as shown in Fig. 2. Generally, Fe, W, Cr, Co and Cu were regarded as impurities that can significantly accelerate the corrosion of Mg alloys. Fe, W, Cr, Co and Cu can support very large cathodic hydrogen reduction current density, as revealed in Fig. 2. For Fe, W, Cr, Co and Cu, the hydrogen reduction ability of Cu is the weakest, which is about 1/10 of other 3 elements. Ag is an interesting element. Although the corrosion potential of Ag was the highest in the studied metals, Ag only showed the medium ability of supporting hydrogen reduction. The cathodic current density of Ag at −1.7 VSCE was about 3.4 mA/cm2. Ti and Zr showed very weak cathodic hydrogen reduction ability, with only about 0.25 mA/cm2 cathodic current density at −1.7 VSCE. Ti has very low solid solubility in Mg and it does not form second phase particles with Mg. Ti can be used as a grain refiner for some Mg alloys. The weak hydrogen reduction ability of Ti and its low solid solubility in Mg indicate the galvanic corrosion effect of Ti in Mg almost need not be considered. Zr is the most common grain refiner for Al-free Mg alloy, whose content is generally below 0.6 wt% [18]. This indicates a small amount of Zr, as long as it does not combine with other elements to form the second phase, should not be a problem in terms of galvanic corrosion with Mg. Zn, Sn and Al are common alloying elements for Mg alloy. They are generally added as the main alloying elements to obtain good mechanical properties [19,20]. Their content in Mg is generally between 1 wt% to 10 wt%. The hydrogen reduction ability of Zn, Sn and Al is relatively low. The cathodic current density for Zn, Sn and Al at −1.7 VSCE were about 0.65 mA/cm2, 0.45 mA/cm2 and 0.3 mA/cm2, respectively. In summarize, Ti, Al, Sn, and Zr have lower cathodic current density, while Fe, Cr, Co and W behave inversely. As a consequence, it is recognized that Ti, Al, Sn, and Zr have weak hydrogen evolution ability and can be alloyed with magnesium in terms of corrosion property. Oppositely, Fe, Cr, Co and W are strong in hydrogen evolution and can accelerate the galvanic corrosion of magnesium alloys significantly, which should be avoided even as impurities.
3.2 Anodic polarization tests
Anodic polarization was performed on pure Zn, Al, Ag, and Sn (Fig. 3) as they have relatively large solid solubility and are often used as main alloying elements in Mg alloy. Performing this test is to indirectly check if they can provide protective effect on the corrosion of Mg alloy when they are oxidized to corrosion products. Zn does not show passive behavior, as the current density increases stably with increasing potential. But Ag, Sn, and Al show passive effect and the breakdown potential can be seen on the anodic polarization curves. The potential ranges of passive region for Al, Sn, and Ag are 430 mV, 250 mV, and 110 mV, respectively. This indicates the protective effect of the oxides of alloying elements might be in the following order Al > Sn > Ag. The enhanced passive effect due to alloying with Al, Sn and Ag were also observed in Mg alloys [21–24]. This is consistent with our study. Some work also reported that Zn alloying can improve the stability of corrosion film of Mg alloy slightly [25,26], which is not fully in agreement with our study on pure Zn. However, by considering the relatively strong cathodic role of Zn and Zn-containing phases and the weak or no passive effect of Zn-containing film, it is reasonable to infer that alloying with Zn could not improve corrosion resistance of Mg.
3.3 Galvanic corrosion tests
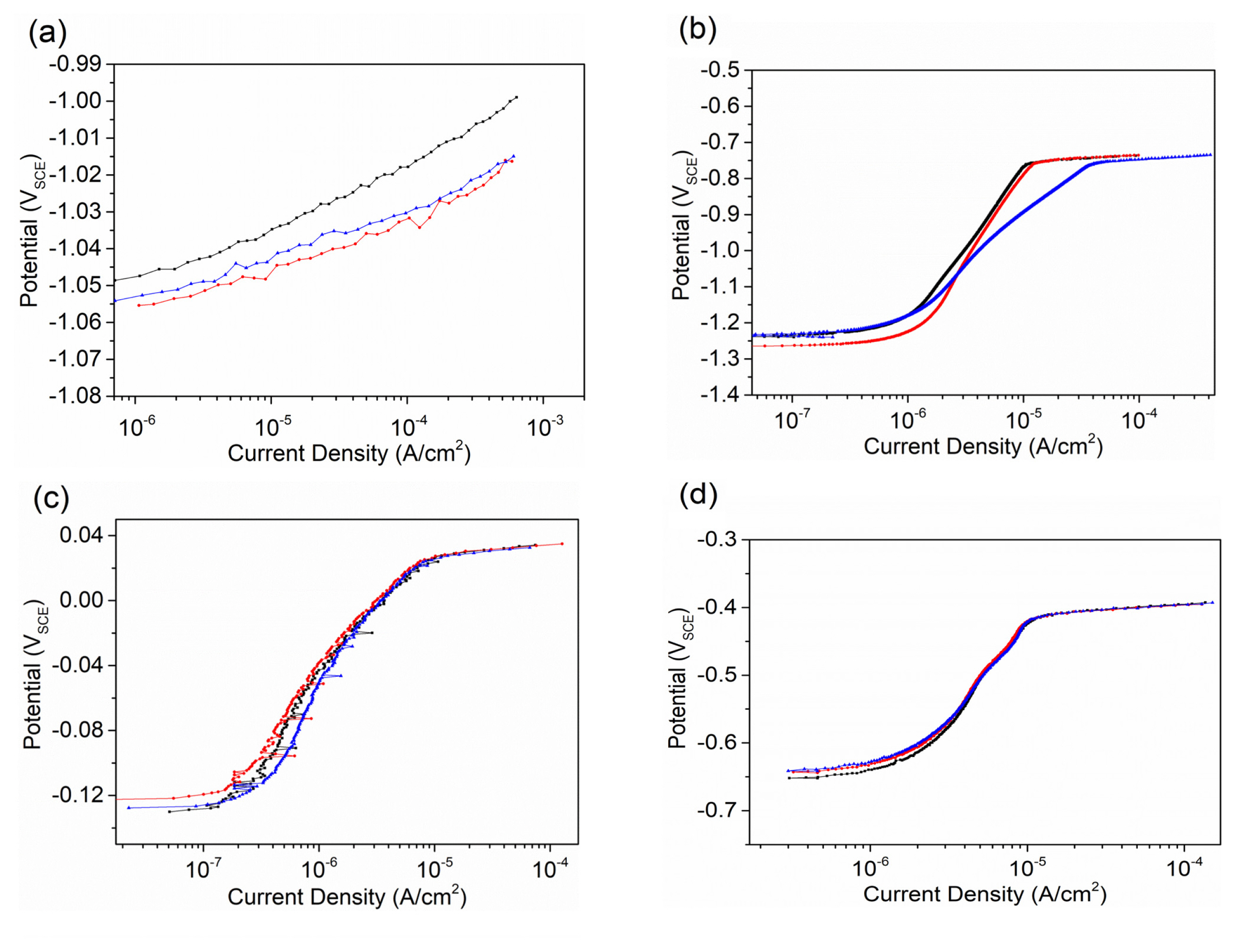
The curves of potential and current density variation as a function of time during galvanic coupling between Mg and other metals are presented in Fig. 4. The mixed potential of all the galvanic couples only varies slightly, around −1.68 VSCE. Thus, the potential is considered to be similar and its effect on current is ignored. The initial current density for all the galvanic couples was similar to the value obtained by the potentiodynamic polarization test. However, with increasing time, the current density varied differently for different metals, which indicates the initial cathodic polarization behavior could not completely reveal the long term galvanic corrosion behavior between Mg and other metals. The variation of current density on pure Sn was relatively small, which decreased initially and then increased, and finally kept relatively stable at about 0.7 mA/cm2. It was also been studied by Cain et al. [20] that Mg-Sn alloys have lower corrosion rates when compared to high purity Mg, especially for Sn addition of 10%. This indicates Sn does not generate strong cathodic hydrogen evolution effect, which agrees with our work. For Zr, Cu, and Ag, the current density increased initially and then reached a relatively stable value after about 2.5 hours. The increased percentages for Zr, Cu, and Ag are about 50%, 100%, and 200%, respectively. For Ti, the current density increased very fast in the first 20 minutes and then increased slowly but linearly, which increased by 300% and was still increasing after 20 hours. Therefore, the enhanced cathodic activity should be considered when alloying with Ag and Ti during long term corrosion. The accelerating corrosion behavior is also reported in Ag-containing alloys [23,27].
For Zn and Al, the current density decreased with increased time and then kept stable. However, the decreased degree has a large difference. The stable current density for Zn was about half of the current density initially. Yim [28] studied the as-extruded TZ alloys and found that both the corrosion potential measured by potentiodynamic test and the current density at cathodic region increased with increase of Zn content. It means Zn content should be controlled strictly in magnesium alloys though the current density of Mg-Zn galvanic couple is low. But the stable current density for Al was only about 1/50 of the initial value. This indicates Al is not only a weak cathode but also has the potential to significantly reduce its cathodic role when alloying in Mg, which is quite interesting. However, the reported corrosion behavior of Mg-Al alloys is very complexed. Bland [29] et al. studied the Mg-Al alloys with ≥ 3 wt% Al content and found that deeper local corrosion occurs. The current density of the alloys is high in cathodic polarization test. This is not consistent with our study. According to our result of Mg-Al galvanic corrosion test, such low current density equals to pretty good corrosion resistance. Therefore, we deduce that impurity elements, such as Fe, may be the cause for the high corrosion rate of the reported Mg-Al alloys [27]. Effects of Fe in magnesium alloys have been studied and it is confirmed that Fe can significantly increase corrosion rate by forming Fe-rich particles in magnesium alloys [30–32]. Generally, less than 9 wt% Al is alloyed in Mg in commercial Mg alloys [27,33]. Based on our study, higher amount of Al is suggested to be alloyed for new alloys development in terms of long term corrosion resistance.
4. Conclusions
The hydrogen evolution ability of selected pure metals and their long term galvanic corrosion behavior with pure Mg were studied by polarization and galvanic coupling tests in the present work. The hydrogen evolution ability of pure Ti, Al, Sn, and Zr is low at the general corrosion potential of Mg (−1.7 VSCE). Thus, they can be alloyed for the development of corrosion resistant Mg alloys. Fe, Co, Cr, and W should be avoided for obtaining acceptable corrosion resistance. Cu, Ag and Zn could be used as alloying elements if high corrosion resistance is not priority. Long term galvanic tests indicate cathodic inhibition is significant for pure Al, while slight cathodic activation occurs for Zr, Ti, Ag and Cu.