1. Introduction
2. Experimental
2.1 Materials
2.2 Electrochemical measurements
2.3 Analysis of corrosion products
3. Results
3.1 SEM/EDS analysis of corrosion products
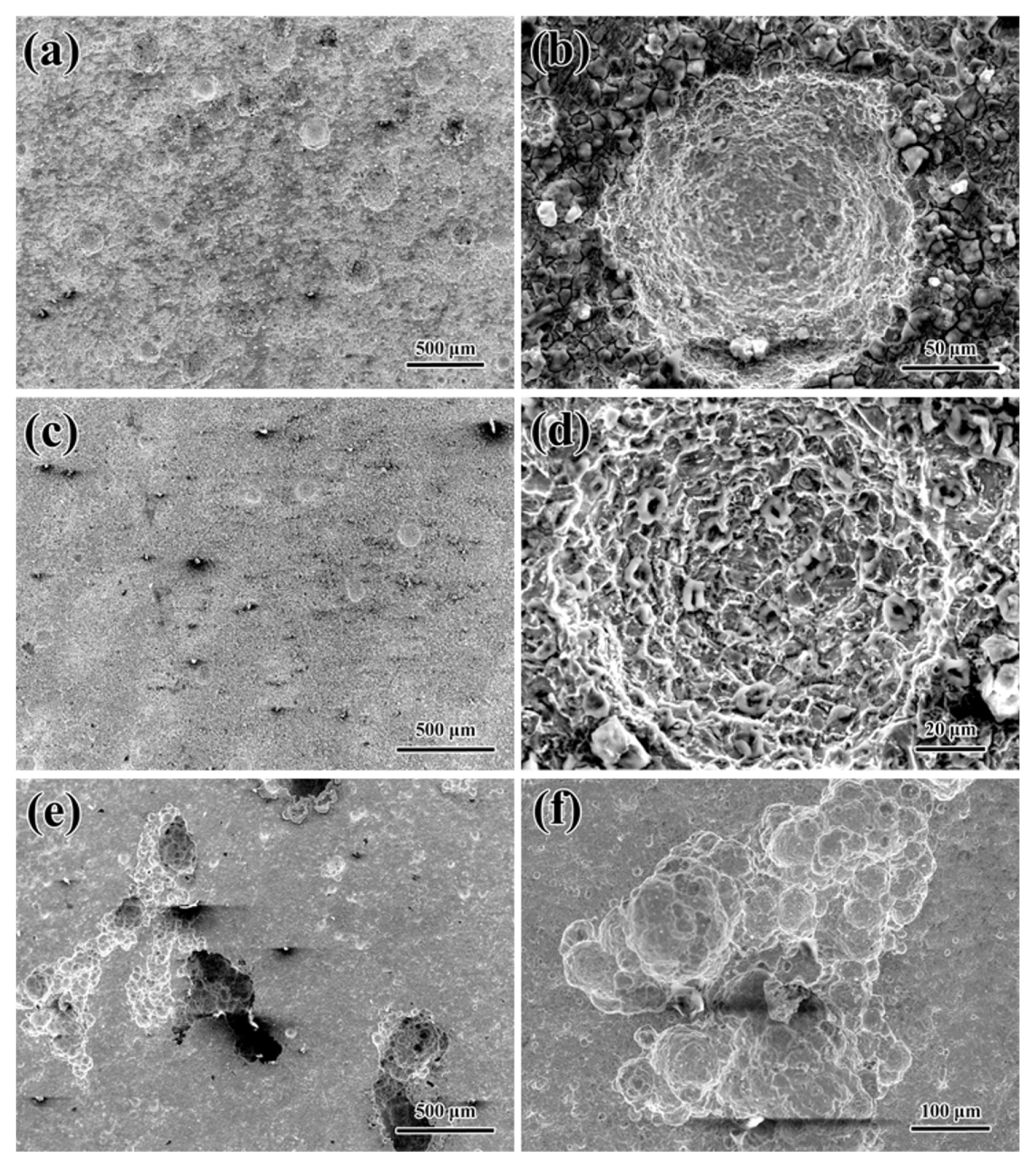
3.2 SEM analysis of corrosion morphology
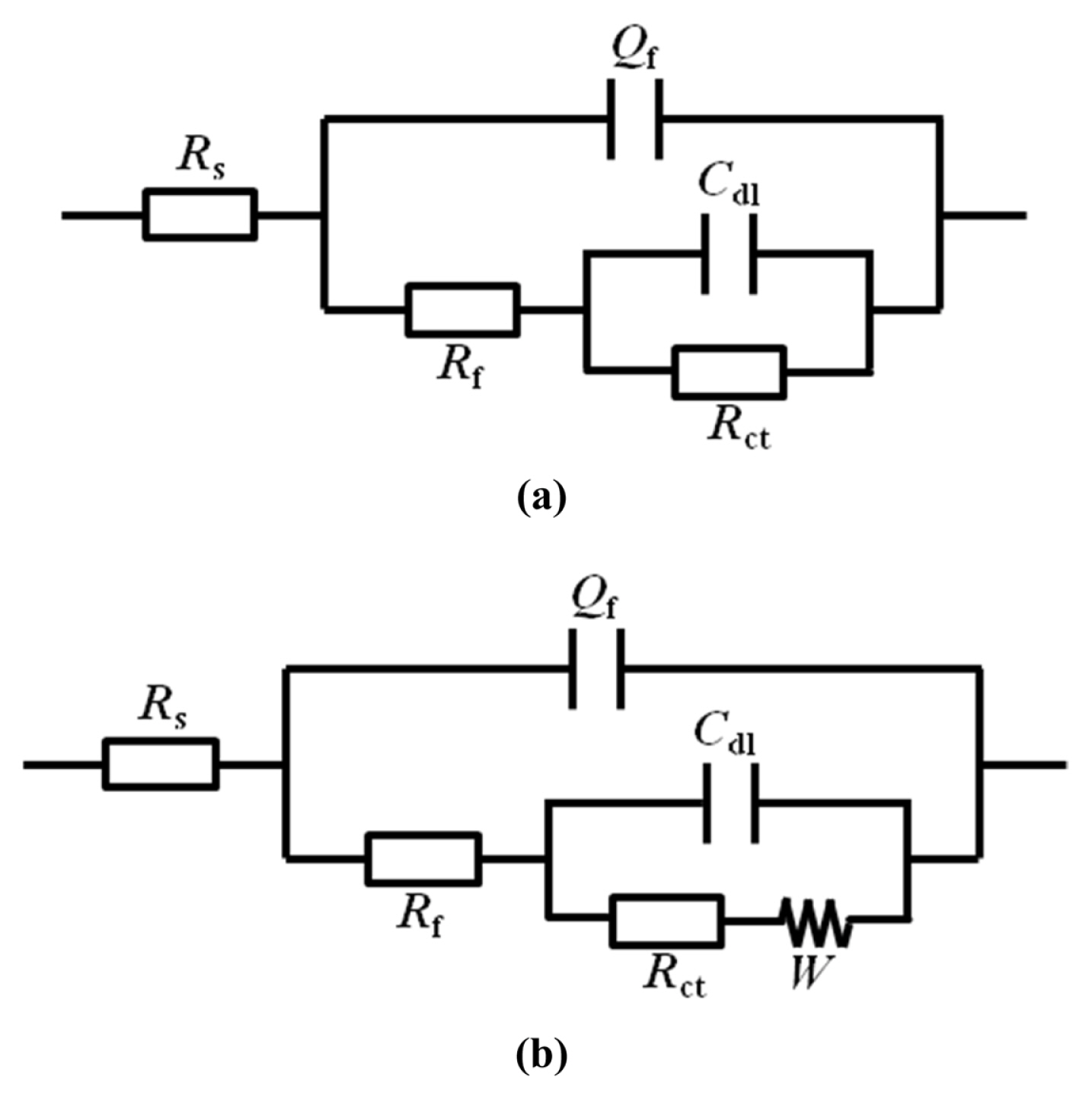
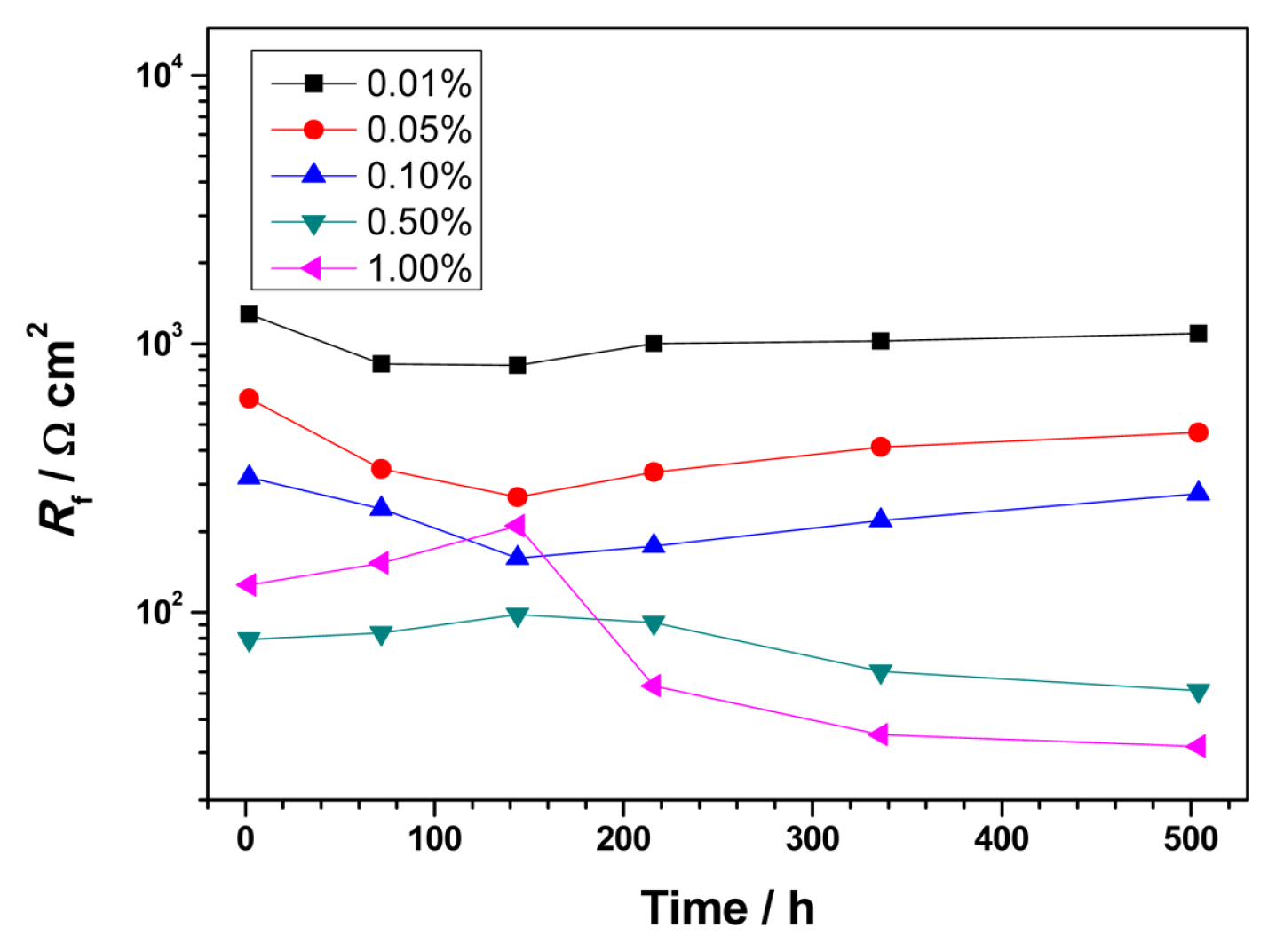
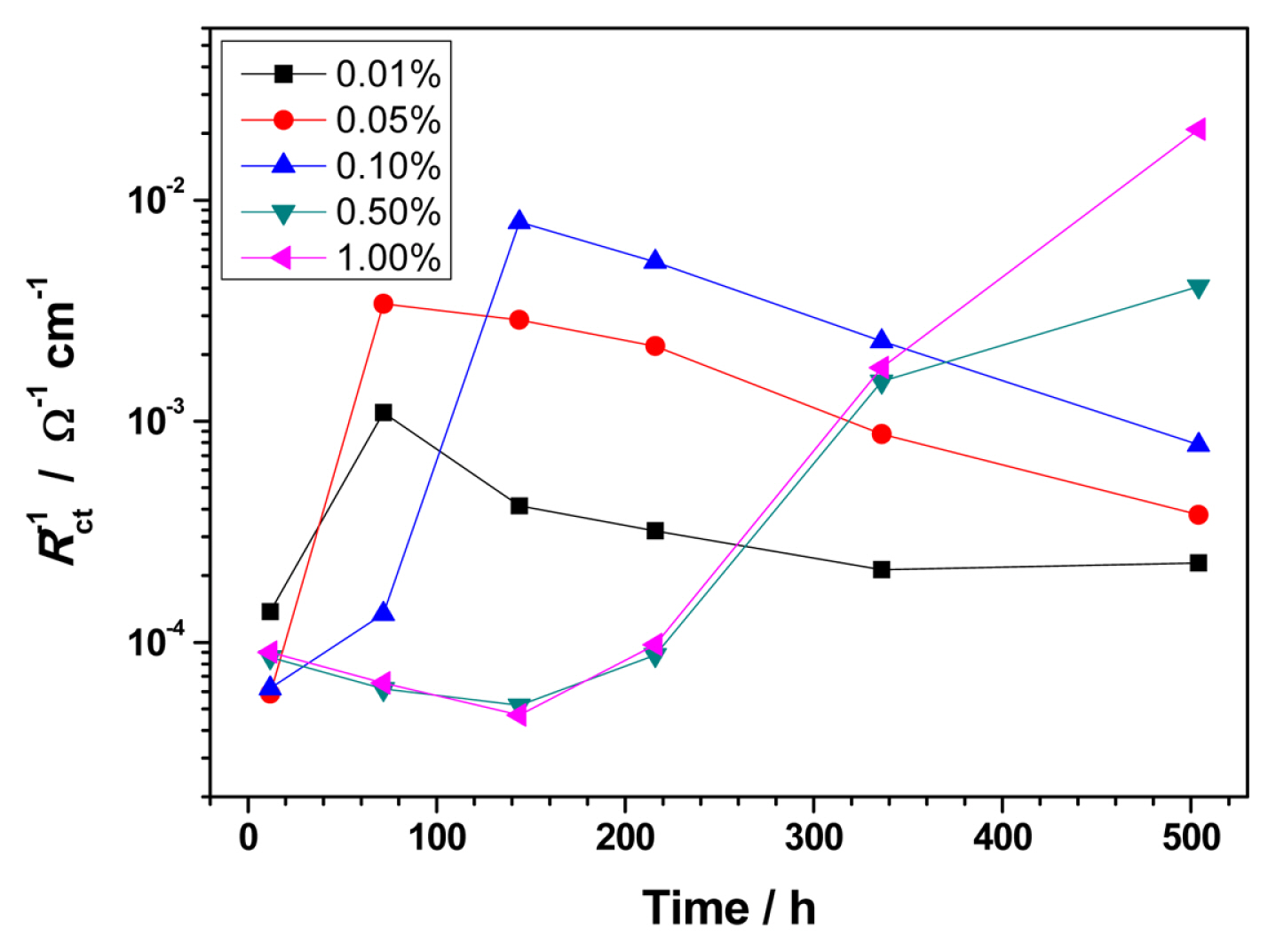
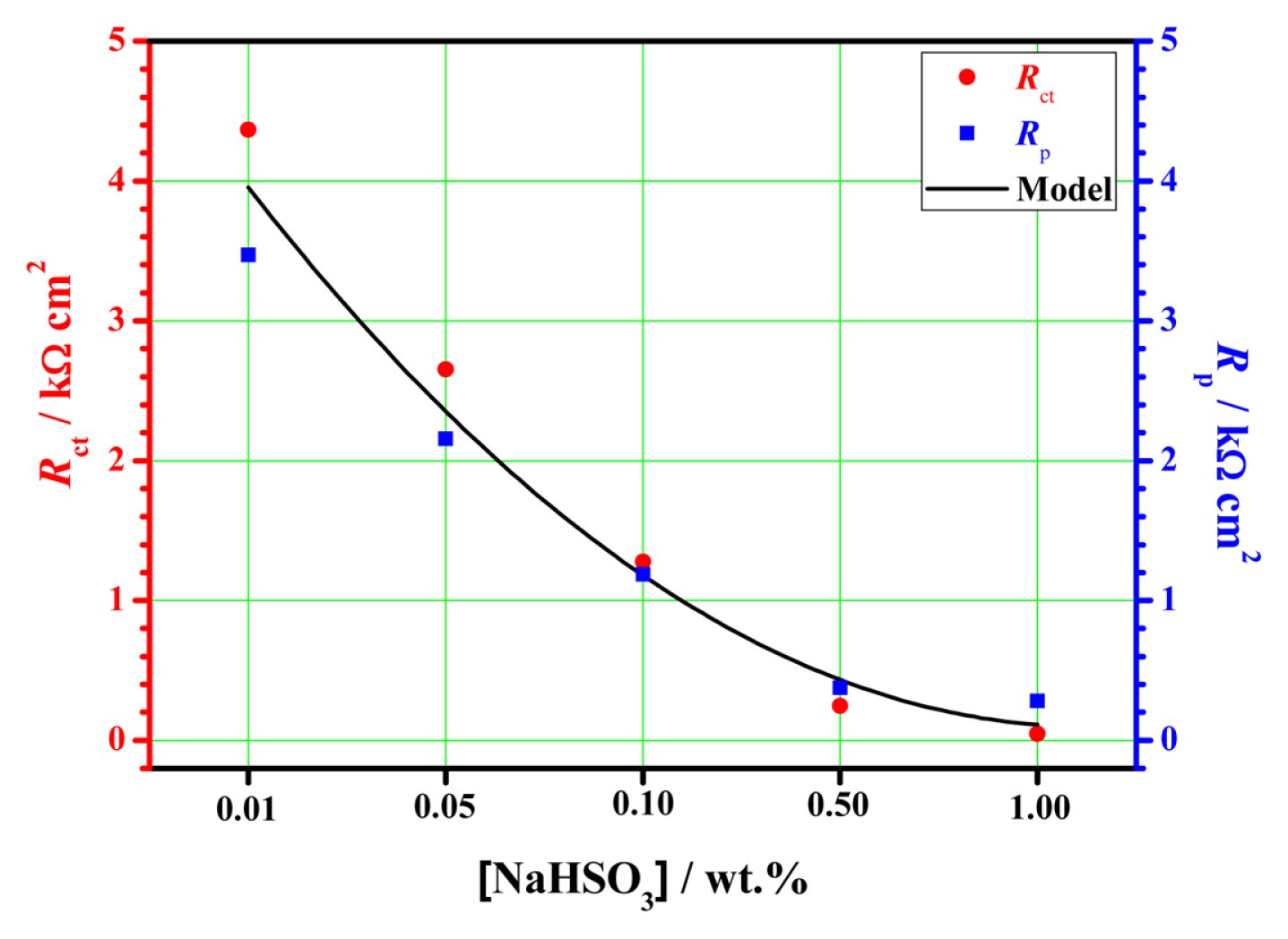
3.3 Electrochemical impedance spectroscopy (EIS)
3.4 Tafel polarization curve
4. Discussion
5. Conclusions
After immersing in NaHSO3 solution for 504 h, intact corrosion products can be generated on the steel surface. With the increase of NaHSO3 concentration, the sulfur content in corrosion products increases, but the compactness and mechanical properties of the corrosion products decrease accordingly.
In the low-concentration NaHSO3 solutions, the steel tends to undergo uniform corrosion with slight corrosion spots. But, as the NaHSO3 concentration increases, the localized corrosion sensitivity of the steel increases, and the corrosion model gradually changes into localized corrosion.
In low- and high-concentration NaHSO3 solutions, Rf of the steel exhibits different evolution patterns, and the corrosion behavior of the steel is almost opposite.
The steel in the NaHSO3 solutions exhibits an obvious characteristic of active anodic dissolution. The cathodic and anodic polarization behaviors are all directly affected by the NaHSO3 concentration, and the polarization resistance of the system gradually decreases with the increase of NaHSO3 concentration in solution.