Separation of Cd(II) from Aqueous Solutions by A New Consecutive Process Consisting of Supported Liquid Membrane and Electrodialysis
Article information
Abstract
Supported liquid membrane process usually is used for recovering or enrichment of valuable metals in the industrial wastewater. But, even if the metals in the wastewater was separated with high chemical selectivity, it cannot be enough concentrated since separation performance of supported liquid membrane (SLM) process is limited by concentration gradient between feed solution and stripping solution. If metal concentration in the stripping solution to be enough low, transport of metal through membrane can be accomplishment constantly. Therefore, Electrodialysis (ED) has been placed after SLM process and the stripping solution of SLM was used as the feed solution for the ED process. Transport of ions in the solutions is successfully performed by ED process. Thus, the metal concentration in the stripping solution does not rise as to stop ion transport. Besides, valuable metals easily are concentrated by ED process for re-use. In this study, effects of operation parameters like initial Cd(II) concentration, HCl concentration in the feed solution of SLM and applied voltage are investigated on separation efficiency, flux and permeability of the both processes. As the feed solution concentration increased, all performance values has increased. When initial concentration of 100 mg/L is used, separation performances (SP) are 55% and 70%, for SLM and consecutive process, respectively. The best HCl concentration in the feed solution of SLM has determined as 2 M, in this conditions SP are 64% and 72%, for SLM and consecutive process, respectively. With increased of applied voltage on ED process, SP of the consecutive process has been raised from 72% to 83%. According to the obtained experimental data, consecutive process has better separation performance than SLM. When the separation performances of both processes were compared for the same operating conditions, it was determined higher the separation efficiency, permeability and flux values of the consecutive process, 8%, 9% and %10.6, respectively. Consequently, the use of the consecutive process increases the performance efficiency of both processes. The consecutive process studied has quite a good chemical separation efficiency, and enrichment capability. Moreover, this process requires few water and energy.
1. Introduction
Cadmium is a precious and rare metal that coexists with the zinc ore in the nature and comes into being as a by-product in the manufacturing of zinc. Besides, it is one of the most hazardous metals for the lives of organisms [1,2]. The buildup of cadmium and its various compounds in human body may affect lungs, kidneys and reproductive organs and also may cause carcinogenic and teratogenic effects [3]. However, the use of cadmium still continues in various industries including Ni-Cd battery manufacturing, production of fertilizer with phosphate, pigmentation and dye industry, electroplating, nuclear plants and photography [1,4–6].
Different processes are used for removal of heavy metals in the wastewater. Using these processes such as chemical precipitation, filtration [7], ion exchange [8] and adsorption [9] may cause sludge formation, chemical material requirement, high costs. Furthermore, the metal recovery capacity of these processes is not adequate [10]. Apart from these, the liquid-liquid extraction processes may be used for metal enrichment as well, but the disadvantage of this process is that it requires a great amount of solvents [11]. In recent years, electrochemical methods and liquid membrane systems have attracted more attention than other methods for removing cadmium from waste water. Electrochemical processes have significant advantages such as high ion removal efficiency, possibility of metal recovery and not sludge formation [6,12], while the liquid membrane process has high chemical selectivity for the ions [5,13–17]
There is some study related with using of electrochemical methods in treatment of different wastewaters [18,19]. ED process can be described as the separation of ionized species in the dilute solutions, passing from ion exchange membranes according to their ionic charge under influence of an electrical field [20,21]. Using the ED process, precious metals with low concentration could be recovered and treated water can be used again. [22]. ED process can be used for many purposes such as salt removal from sea water [23], concentration or separation of acids [24], and recovery of metal salts from industrial wastewater [25]. ED process can be installed and operated in a small area without sludge formation and without the requirement of chemical addition compared to other processes [6]. The most important drawback of the ED process is the concentration polarization due to the increase in electrical resistance by decrease of the feed solution concentration in the process [6]. If concentration polarization is realized, ion separation efficiency decreases, energy consumption increases [26]. However, in the high concentration of feed solution, lifetime of ion exchanging membrane may reduce and/or larger scale processes may be required. [22, 26]. Separation efficiency at ED processes considerably depends on applied voltage, flow rate, initial concentration and pH considerably [12].
Liquid membrane processes can be used for recovering and removing precious metals from wastewater through high chemical selectivity. Liquid membranes can simply be described as single step of liquid-liquid extraction and stripping steps which being in the solvent extraction [27]. When complexing agent is used for facilitating transport of ions at the liquid membrane process, the realized transport is defined as the facilitated transport. Important studies related with enriching and separating metals from wastewater through facilitated transport at liquid membrane [15,19,28–30]. In recent years, studies related to liquid membranes have focused on supported liquid membranes. In supported liquid membrane processes a solvent including complexing agent (carrier) is immobilized into the support material with porous (polymer etc.) [1,16,28,31]. In many studies conducted on the separation of Cd(II) ions through SLM process has been used Aliquat336 as carrier [17,24,32].
Efficiency of ion transport in SLM process is affected by concentration difference between feed and stripping solutions. When the metal concentration is increased in the stripping solution as a result of the transportation of metal from feed solution to stripping solution, concentration difference between stripping solution and feed solution is decreases by time. As a result of this, transfer of ion at SLM process decreases and then stop. Even if metal is transported through chemical selectivity, the desired concentration is not reached; and in this situation, the stripping solution has to re-newed [19]. If concentration of stripping solution can be kept low in a continuous manner, the substance transport may continue since the concentration difference will continue as well.
There are a few consecutive studies including complexing agent, liquid membranes and electrochemical methods in literature [10,33–35]. But, there is yet to be found another study in which two processes are used consecutively as is done in this study.
In this study, the membrane prepared by impregnation into porous polymer membrane support (PVDF) of toluene containing Aliquat336 had been used in SLM. Properties of this membrane is suitable for separation of Cd (II) according to previous study [19].
The aim of this study was, firstly, removing continuously by ED process of Cd(II) ions in the stripping solution to keep of concentration difference between feed and stripping solutions which affected process efficiency. To this end, ED process, which reached high separation efficiency even in low feed concentrations, was used after SLM process. Within the scope of this study, the separation with chemical selectivity of Cd(II) from a solution consisting of Cd(II) is investigated at both SLM process and SLM-ED consecutive process comparatively.
The effects on process performance values of initial concentration and acid concentration of the feed solution are determined for both processes. In addition, the influence of applied voltage on the ED part of the consecutive process was investigated as well. As a result, SLM and consecutive processes were compared.
2. Experimental
2.1. Materials
Aliquat 336 (Methyl-tri-caprylyl ammonium chloride) (Aldrich Chemical Company) was used as carrier. High purity grade toluene (Merck chemical company) was used as a membrane solvent. All of chemicals (CdCl2·5H2O, HCl, NaCl) that were used for the preparation of other solutions had the highest analytical purity grade. A hydrophobic PVDF (Polyvinylidene difluoride) membrane (DuraporeTM) that had a pore size of 0.22 μm, a thickness of 120 μm and a porosity of 60% was used as a support material to hold the membrane solution containing carrier. NaCl was used as electrolyte solutions, Nafion117® as cation exchange membrane (CEM), Neosepta® as Anion Exchange Membrane (AEM).
2.2. Experimental Methods
Supported Liquid Membrane;
PVDF hydrophobic membrane (40×70 mm) saturated by toluene containing Aliquat336 was used as membrane in SLM. The membrane was placed between the two half cells of the module and fixed in the SLM cell. During the experiments, the volume of solutions circulated from each part was 1 L in quantity. Throughout the runtime of two hours, 1 mL samples were taken from each solution periodically (0, 15, 30, 60, 90 and 120th min.).
Electrodialysis Processes;
3 compartment microflow laboratory-scale ED module was used with Pt/Ti electrodes that was provided by the company named Electrocell®. The effective area of the membrane is 10 cm2. All of solutions was circulated into the compartments using a multi-head peristaltic pump (Longer Pump WT600-2J).
Consecutive process was formed by consecutive running of processes mentioned above. The stripping solution of SLM was used as the feed solution for ED (Fig. 1). After each experiment by applying reverse current (15 min.), distilled water was passed from feed compartment of ED for cleaning the ion exchange membranes. During the run-time, samples of 1 mL were taken periodically (0, 15, 30, 45, 60, 75, 90, 105 and 120th min.) from all solutions and then Cd (II) was determined with an Atomic Absorption Spectrophotometer (AAS) that is Thermo Scientific Ice 3000 Series model.
All experiments were performed at ambient temperature. All solutions circulated were stirred with a digital magnetic stirrer (Heidolph MR 3004S) throughout the experiments.
2.3. Analysis of experimental data
Performance values of the processes was calculated using the following equations. Ion transfer in the SLM process was explained by Fick’s First Law [19].
Here, J is the flux of process (mol/cm2·s) and P is the permeability value of membrane (cm/s). Permeability of membrane were determined by using Cd(II) concentration in the feed solution as a function of time and was computed by using the following equation.
Here [Cdf] and [Cdf0] are Cd(II) concentrations in the feed solution at the t time and the initial time, respectively. Ae is the effective area.
Separation percent (SP%)
3. Results and Discussion
In the experimental studies, an amount of 0.01 M HCl was used as the strip solution and 0.01 M Aliquat336 as carrier and at the flow rate of 80 mL/min. For ED process, the flow rate is 200 mL/min; and electrolyte solution is 0.05 M NaCl. To reveal the contribution on Cd (II) transfer of consecutive process, the same operation conditions were performed for both SLM process and consecutive process.
3.1. Influence of initial Cd (II) concentration
Experiments were performed for different initial concentrations (25, 50, 75, 100 mg/L) of the feed solution. In those experiments, the voltage applied to ED process was 40 V. The performance values for both process are presented in the Table 1.
According to Eq. (1) flux is a function of initial concentration. As a result of Eq.(1), as seen in Table 1, separation efficiency, flux and permeability values increase as a result of an increase in the concentration of the feed solutions for both processes [29]. However, performance values of consecutive process were higher. The temporal changes of Cd (II) ions in the solutions of both the processes for the 100 mg/L feed solution, where the highest performance values were obtained, are presented in Fig. 2.
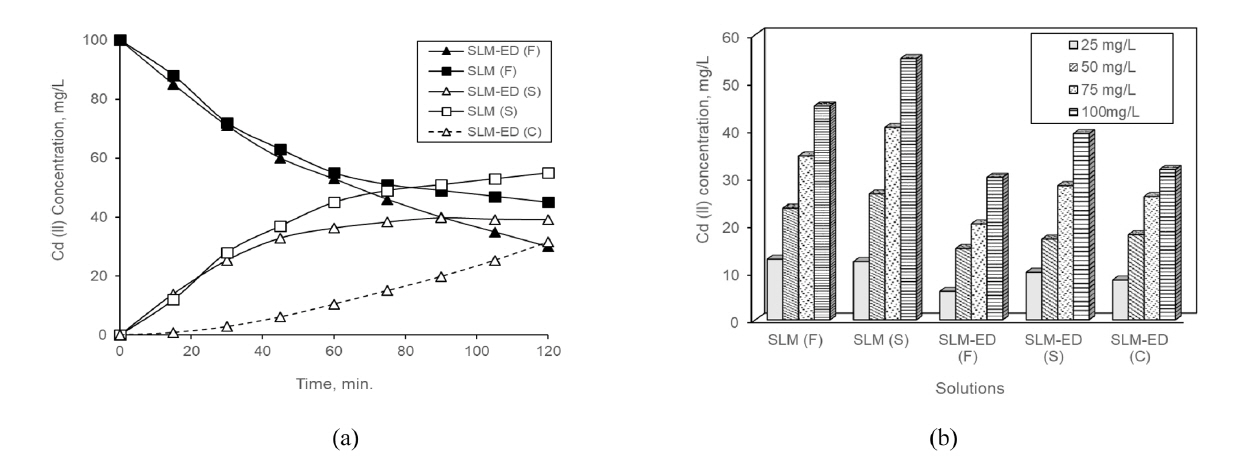
(a) Cd (II) concentrations in the processes having initial concentration of 100 mg/L for the feed solutions (b) Cd (II) concentrations in solutions of the processes having different initial concentration at end of 120 min. (F; feed, S; stripping, C; concentrate, operation conditions; acid concentration of feed solutions: 1 M HCl, applied voltage; 40 V).
Considering Fig. 1 it may be seen that Cd (II) concentrations of the feed solutions in the processes are decreased by time. At the end of run-time, 55 mg/L Cd (II) for SLM and 70 mg/L Cd(II) for consecutive processes were transferred from the feed solutions. For consecutive process, while the transferred amount of 39.1 mg/L of Cd (II) stayed in the stripping solution, an amount of 31.6 mg/L Cd (II) was passed to the concentrate solution of ED (Fig. 2).
The Cd (II) transferred to stripping solution has increased by increasing of initial concentration. When considering consecutive process, there seems to be a lesser amount of Cd (II) in the feed and stripping solutions compared to the SLM. Because, a significant portion of Cd (II), which was transferred to stripping solution, was transported to concentrate solution. While the rate of ion transport slowed down in the SLM process, it continued in the consecutive process.
3.2. Effect of HCl concentration in the feed solution of SLM
In SLM process, Cd (II) was transported to stripping solution from feed solution by using Aliquat336 as carrier. Cd (II) creates negatively charged chloride complexes depending on chloride and H+ concentration of feed solutions. These negatively charged complexes are replaced by the chloride present in the Aliquat336 at appropriate pH values [11,19]. For this reason, the Cl− and H+ content inside the feed solution has an effect on the transportation of Cd (II). The effects of HCI concentration in the feed solution on transport were identified separately for both the SLM and SLM-ED processes. The tests were conducted by using feed solutions that contained 100 mg/L Cd (II) at different HCI concentrations (0.5 M, 1.0 M, 2 M). The voltage applied to ED was 40 V.
With the increase of HCI concentration in the feed solution, a larger amount of Cd (II) was transported due to the increasing Cl− concentration and decreasing pH value [19]. According to the obtained performance data, the effects on flux and separation efficiency of HCl concentrations are similar, but the use of consecutive process led to an enhancement in all performance values (Table 2). The best performance values were obtained when 2M HCI concentration was used.

The performance values determining for the processes when feed solution is prepared by the different concentration of HCl.
According to Fig. 3 a and b, concentration Cd (II) resting in the feed solution is 36 mg/L for SLM and 28 mg/L for consecutive process. Approximately, half of Cd(II) which was transported in the consecutive process was carried to the concentrate compartment of ED.
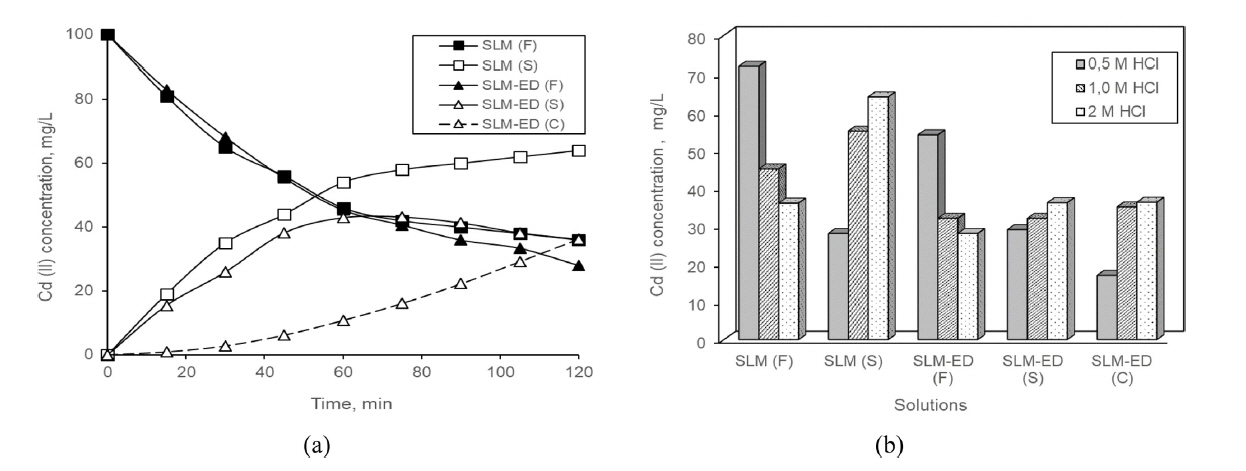
a) Cd(II) concentrations at the processes which prepared with 2 M HCl of the feed solutions. b) Cd(II) concentrations in solutions of the processes having different acid concentration in the feed solutions at end of 120 min. (F; feed, S; stripping, C; concentrate, (operation conditions; feed solution concentration 100 mg/L, applied voltage 40 V).
According to these results the separation performance of consecutive process is better than SLM under the same operating conditions. In the consecutive process, stripping solution of SLM is feed solution for ED at the same time. That is, increasing the concentration of Cd (II) in the stripping solution is mean to rising of concentration of feed solution at ED.
Separation performance of ED process by increasing of feed solution concentration in the ED processes increased as well [12]. Also, to be high of solution concentration in both concentrate and dilute compartments at ED processes provide easier passing of current [6]. For this reason, when concentration in the stripping solution increases, more Cd (II) is concentrated in the concentrate compartment. Operation parameters affecting the separation efficiency in the SLM also effect the efficiency of consecutive process in a similar way. However, consecutive process is always more efficient under the same operation conditions.
Concentration difference between feed solution and stripping solution in the consecutive process is higher than to the SLM process. Thus, high concentration difference obtained in consecutive process provides the continuance of the ion transfer.
3.3. Effect of applied voltage
Different voltages (30, 40 and 45 V) were applied with the aim of determining the suitable voltage for consecutive process. When different voltages were applied to the ED that is part of the consecutive process, performance values were obtained as shown in Table 3. The Cd (II) concentration in the concentrate compartment for different voltages are presented in Fig. 4.
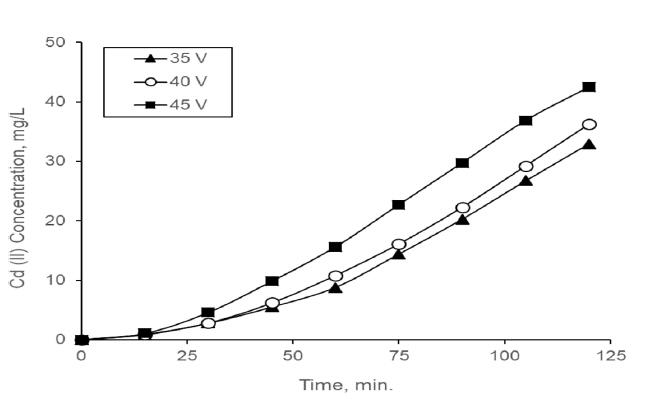
For different voltages in consecutive process, the changing of Cd(II) at concentrate compartment along runtime. (Feed solution Cd (II) concentration; 100 mg/L and HCl concentration of feed solution; 2M HCl)
The driving force behind the mass transfer in ED processes is the electrical current and with this reason the separation efficiency and flow efficiency at the ED increase with increasing of the voltage applied up to reaching the limiting current density. That is, performance of ED process is better when it was operated at near and below the limiting current density [6]. High current density facilitates overcoming resistance in the membrane and boundary layer [36]. If ED process is operated under the limiting current, fouling and concentration polarization may be prevented or delayed. As seen Fig. 2 Cd (II) transferred to concentrate compartment was increased with an increase in the applied voltage.
3.4. Comparing of SLM and consecutive process at optimum operation conditions
As a result of the experimental studies, optimum operating conditions (Feed solution Cd (II) concentration; 100 mg/L and HCl concentration of feed solution; 2M HCl, voltage applied; 45V) were determined for both processes. When then processes were operated in these conditions, Cd(II) concentrations in the all solutions were given during run-time in Fig. 5.
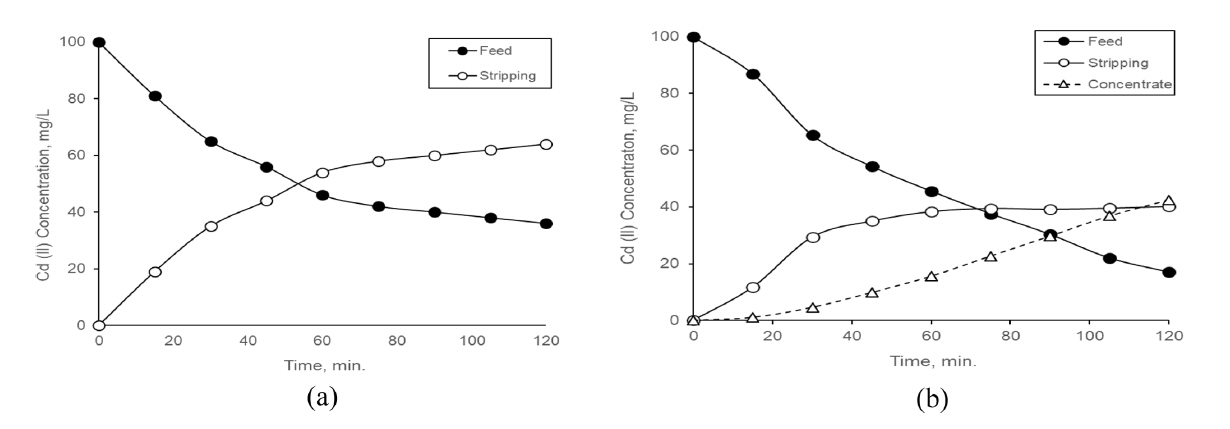
At the optimum operation conditions, changing of Cd(II) concentration in the all solutions of the processes during run-time, (a) for SLM, (b) for consecutive process.
When the performance values of SLM and consecutive processes operated under the same operating conditions were compared, it was determined that the separation efficiency, permeability and flux at consecutive process increased by 8%, 9.7% and 10.6%, respectively (Table 2). The Cd (II) transfer into stripping solution from feed solution in the SLM process almost stopped. Despite that, the ion transfers into the strip solution from feed solution in the consecutive process continued due to the fact that the Cd (II) in the strip solution was transported continuously into concentrate compartment through ED process (Fig 5.)
Cd (II) separation efficiency reaches 83% for the consecutive process. Nearly half of the separated Cd (II) transferred to concentrate compartment. Using consecutive process, Cd(II) was separated with chemical selectivity by SLM, and enriched by ED process.
Thus, while the stripping solution in the SLM can be used for a longer time, the ED process can be operated at ohmic conditions for a longer time without the current efficiency decrement by time because a solution including ion will be fed continuously to ED.
4. Conclusions
A consecutive process, consists of SLM and ED processes has been used in this study. Disadvantages of both processes has been overcome at this new process, better chemical separation and metal enrichment (or concentrating) has been successfully performed.
In the studied consecutive process, firstly the Cd(II) ions are taken into the stripping solution with chemical selectivity from feed solution. Then this stripping solution containing Cd(II) at a low concentration is given to the ED as feed solution. Thus, Cd(II) ions in the stripping solution are transferred to the concentrate compartment of the ED process. Since a solution containing Cd (II) ions pass through the ED process constantly, dilute compartment resistance does not occur against the passage of flow through the process, and thus the Cd (II) ions can be transported with high-flow efficiency. Besides, the ED process may be operated for a longer period without reaching limiting current density. Thus, high current efficiency and low energy consumption can be provided.
As seen from the results, consecutive process has higher efficiency than SLM in all operation parameters. Moreover, although ion transport in the SLM decreased during the run-time, it continued in the consecutive process. The Cd(II) that was separated through chemical selectivity in the SLM part of consecutive process can be collected in smaller volumes by rendering them concentrated in the ED part. Stripping solution in the SLM process can be used with closed circulation without changing for a long time. In this way, Cd (II) can be separated successfully using less volume stripping solution than necessary for conventional SLM process. And then it can be enriched in the ED process.
Using our purposed consecutive system; Separating performance of SLM is increase. in the same operation time more metal is transported. Membrane material and carrier agent requirements of SLM is decrease and operation costs reduced. The life of membrane material is extended. While Cd(II) and other valuable metals are separated by chemical selectivity, in same time it can be recovered by concentrating. ED process is performed without reached to limiting current density. As result of this, efficient using of energy is provided.
Acknowledgement
This research was financially supported by the Research Fund of Bulent Ecevit University (Project code: 2014-77047330-07).


