Electrochemical and Safety Performances of Polyimide Nano fiber-based Nonwoven Separators for Li-ion Batteries
Article information
Abstract
In this study, cell performance and thermal stability of lithium-ion cells with a polyimide (PI) separator are investigated. In comparison to conventional polyethylene (PE) separator, the PI separator exhibits distinct advantage in microporous structure, leading to superior reliability of the cell. The cells with PI separator exhibit good cell performances as same as the cells with PE separator, but their reliability was superior to the cell with PE separator. Especially in the hot-box test at 150 and 180℃, PI separator showed a contraction percentage close to 0% at 150℃, while the PE separator showed a contraction percentage greater than 10% in both width and length. Therefore, the PI separator can be the promising candidate for separators of the next generation of lithium-ion battery.
1. Introduction
A lithium-ion rechargeable battery has been used and increased in the electric vehicles including the hybrid electrical cars. In this application, as we know, lithium metal [1-5], a highly-reactive anode material, may cause an explosion if it comes into direct contact with the cathode [6-12]. Therefore, an electrically insulating membrane to separate the anode from the cathode is critical component for the safety of the lithium-ion battery (LIB). However commercialized LIB has usually adopted a polyolefin type membrane for its separator. However, thermal contraction coupled with physical damage occurs when polyolefin is exposed to high temperatures over 100℃ due to its physical properties and manufacturing process, which could result in a possible short-circuiting inside a battery.
There have been numerous studies to overcome these problems related to current polyolefin separators [13,14]. The PE-based separators have been used in conventional lithium batteries. The PE separators have good mechanical properties, and also they can effectively prevent thermal runaway that results from short-circuits or rapid overcharging of the battery. But, they exhibit poor compatibility with liquid electrolytes due to their hydrophobic property, and their manufacturing cost is high. Many studies of coating on the PE separators with a gel electrolyte have been undertaken to enhance their compatibility with liquid electrolytes [15-18]. The rate capabilities of the separators are not enough for high-power applications, such as electric vehicles, hybrid electric vehicles and robots. The micro-porous membrane separators have some disadvantages that need to be improved, such as lowwettability, low-porosity of about 40% [19], and thermal shrinkage that causes short circuits between electrodes under unusual heat generation. Recently, various approaches to overcome these shortcomings of polyolefin based separators have been reported.
The PI separator has been widely used in many advanced technology fields due to their excellent thermal stability, outstanding mechanical properties, low dielectric constants and inertness to solvent and radiation resistance [20-24]. In spite of this physical drawback, the PI separator has been regarded as effective for battery’s thermal safety, as the demand for large-capacity batteries for electric cars has significantly increased. In this study, an optimal evaluation system for current PE separator and PI separator was come up with, and corresponding stacking-type pouch batteries were designed. Batteries with PE separators were electrically, thermally, electrochemically and chemically compared to those with PI separators.
2. Experimental
PAA (polyamic acid) solution was prepared using by a diluent of DMAC (dimethylacetamide) for electrospinning. Viscosity of PAA solution was 5,000 cps. Electrospinning was conducted at a temperature and humidity of 25℃ and 25%, respectively. And then calendaring was done at a temperature and pressure of 130~140℃ and 10 kg·cm−2, respectively. Thermal imidization using PAA separator was done using a high temperature furnace: increase the temperature at a rate of 5℃·min−1 until 350℃, maintain at 350℃ for 30 min, and decrease to room temperature. PI separators provided by Finetex EnE, Inc. were stacked by a stack-fold method to make full-pouch cells with the design capacity of 1,000 mAh. Before making the full cell, the design specifications for the anode/cathode/electrolyte were taken into consideration. The core anode material is natural graphite with core shell type, while the surface of natural graphite is coated with amorphous carbon layer. In addition, SBR/CMC water-based binders were added to attain a loading level (20 mg·cm−2) per unit area and electrode density of 1.51 g·cc−1. Typical high-output active materials such as Li(Ni1/3Co1/3Mn1/3)O2 (LNCMO) were used for the cathode, while super-P and PVDF binder were used as a conducting agent and a binder, respectively. The cathode was designed to attain a corresponding loading level (39 mg·cm−2) and electrode density (3.64 g·cc−1). The electrolyte specifications were set at 1 M LiPF6 + EC/EMC (1:2 v/v) + VC 2 wt.%. Furthermore, PE separators (Celgard Co. Ltd.) with similar thickness (~18 μm) were compared to PI separators (~19 μm) in order to evaluate each separator type’s performance. In order to establish a full-cell evaluation, the design specifications for a corresponding battery were set at an n/p ratio of 1.1 and a current density of 2.5 mA·cm−2.
FE-SEM (Hitachi Co. S-4800) was used to observe the structures of PI and PE separator, and each separator’s electrochemical reliability, electrochemical stability and thermal stability were accordingly evaluated. For the evaluation of thermal stability, each separator was positioned between slide glasses and subjected to certain tensile shear stress. Then, the temperature was raised to 150℃ and 180℃ at 5℃·m−1, and both separators were kept for 12 h at each temperature in order to compare their contraction percentages.
For evaluation of electrochemical reliability, the related charge/discharge performances were measured in a voltage range between 3.0 V and 4.2 V (by CC (constant current) - CV (constant voltage)) on charge/discharge curves. Rate capability was evaluated at a current of 0.2 C charge and 0.1 C discharge. Also, the cyclic durability was measured at room temperature (25℃) and high temperature (45℃) while the C-rate was set at 1 C. For the analysis on electrochemical properties of each separator, high-rate discharge capacity was evaluated and discussed with physical properties of separator. The high-rate discharge capacity was also tested with 0.2 charging and 1 - 10 C discharging between 2.75 V and 4.2 V.
For the evaluation of electrochemical stability during cell operation, the LSV (linear sweep voltammetry) analysis was conducted on each separator. The measurement was performed in a two electrode electrochemical system consisting of a SUS (stainless steel blocking electrode) working electrode, and lithium counter electrodes at room temperature. LSV was scanned between 0 V and 5 V at a rate of 2 mV·s−1. The full cell with each separator was fully charged up to 4.2 V under CC/CV trickling charging mode and then the surface of each separator was analyzed with SEM-EDS (Hitachi Co. S-4800).
3. Results and discussion
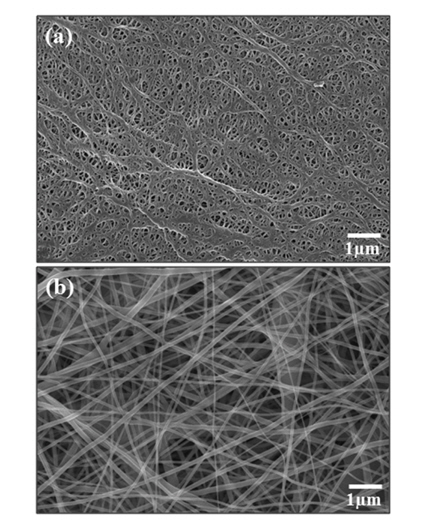
The PE and PI separator have different morphology, as shown in Fig. 1. The PE separator shows a net structure with many open pores and these pores are uniformly distributed. On the other hand, as shown in Fig. 1(b), a number of nano-fibers can be observed by SEM images. The fibers exhibit homogeneous diameters and any observable beads do not exist on the fibers, as same as shown in a polyacrylonitrile based nanofiber [27]. In contrast to a PE separator having large number of small-sized micropores, of which average diameter appears to be below 0.1 μm, the pristine PI separator shows excessively large-sized (0.2~0.5 μm) pores that are arbitrarily distributed between the PI fibers. It is well known that various physical properties of separator are closely related with its morphology. The measured thickness of the PI separator is in the range of 18 μm, of which values are similar to that of the PE separator.
In order to see how the PI separator electrochemically reacts at highly electrochemical oxidation condition, the related tests were implemented from two perspectives. First, in order to observe the oxidation reaction and side reaction at certain high-voltage conditions, a cell with a stainless steel working electrode/separator/electrolyte (with LiPF6, w/o LiPF6)/Li (reference electrode, counter electrode) was made for the LSV test. As shown in the Fig. 2, there was almost no change in the onset voltage, which indicates the sudden occurrence of an oxidation reaction. Then, an LSV test that excluded LiPF6 to remove any oxidation reaction current, which might be triggered by anion in dissociated salt, was conducted. As a result, very little parasitic current was generated for both separators even at high voltages up to 5 V, implying that there was no additional oxidation reaction triggered by the PI separator.
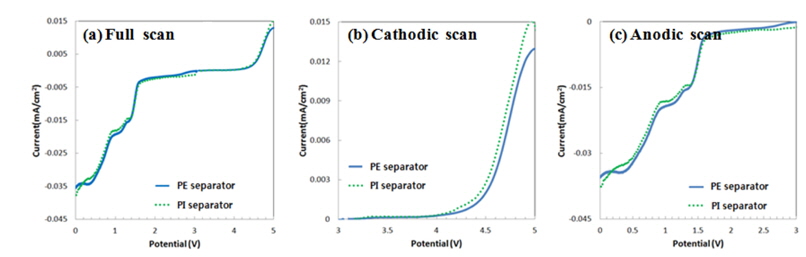
Linear sweep voltammograms of the cell with a stainless steel working electrode/separator/electrolyte at a scan rate 2 mVs−1 at scan voltage of (a) 0~5 V, (b) 3~5 V, and (c) 0~3 V in electrolyte of EC: EMC = 1: 2 (v/v)+ VC 2 wt.% with LiPF6.
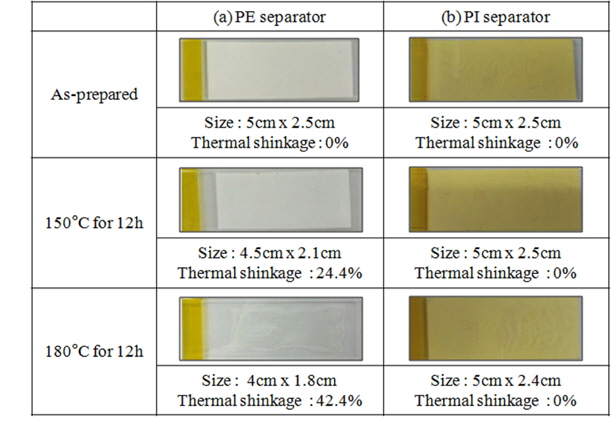
In order to evaluate certain heat-resistant properties of separators developed in this study, test conditions similar to the 150℃ hot-box test (a standard test to evaluate the physical conditions of a jelly roll inside a real battery and its safety) were established. Each separator was positioned between slide glasses and subjected to certain tensile shear stress. Then, the temperature was raised up to 150 and 180℃ at 5℃/m, and both separators were kept for 12 h at each temperature in order to compare their contraction percentages. As shown in the Fig. 3, the PI separator showed a contraction percentage close to 0% at 150℃, while the PE separator showed a contraction percentage greater than 10% in both width and length. Moreover, at 180℃, the PI separator showed a contraction percentage equal to only 0%, while the PE separator showed a contraction percentage greater than 25%.
The performances of batteries with the PE separator and PI separator were investigated. At the first cycle, the cells were charged up to 4.2 V under constant current-constant voltage mode, and then discharged to 2.75 V under constant current mode. The initial charge-discharge cycles were tested with a 0.1 C-rate. The following formation cycle charge-discharge tests were charged up to 4.2 V (0.2 C) under constant current-constant voltage mode, and then discharged to 3 V (0.1 C) under constant current mode. A cell’s design capacity was set at 1,000 mAh. A pouch battery’s 0.2 C standard capacity was measured to be 101~105% of the corresponding designed capacity, and the battery became fully activated (reversible efficiency over 99%) in the second cycle. In addition, the charge/discharge behavior was observed to be almost similar between two separators. Any unstable voltage profiles were not observed for the cells with the both separator as shown in Fig. 4. Both cells shows the similar discharge capacity at the 1st cycle but in case of PI based cell, the reversible efficiency of more than 99% was attained earlier than that of PE’s.
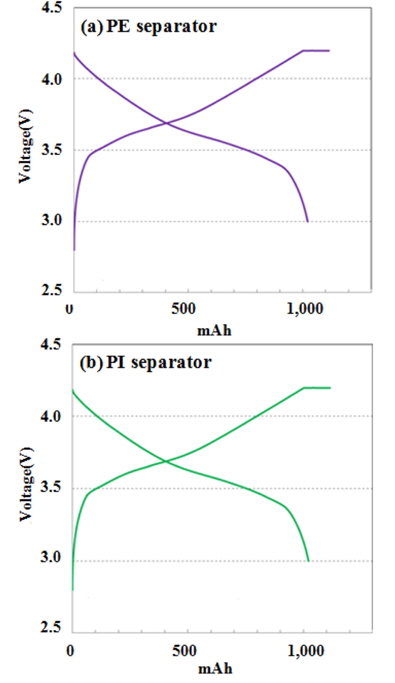
Comparison of charge and discharge curves of pouch type cells with PE and PI separators: at a charge rate of 0.2 C and a discharge rate of 0.1 C.
To observe capacity deterioration behavior of each battery with repeated charge and discharge at 25℃ and 45℃, the electrochemical durability at the 1 C/1 C charge/discharge rate was examined. In terms of the battery life, at room temperature (25℃), both separators showed very similar cycle life without any capacity fading. On the other hand, the newly developed PI separator based battery showed comparatively better durability at high temperature (45℃), as shown in the Fig. 5. To observe the morphological change between the two separators after 100 cycles, each battery was fully discharged at the end of the 100th cycle. Then, each pouch cell was disassembled, and SEM analysis was performed to see how the surface morphology of each separator had been changed (due to oxidation reaction, etc.). As shown in Fig. 6, either separator’s morphology was hardly changed at room temperature. At high temperature, the PE based cell, however, might be observed to be a little gradual deterioration in battery life due to the blocking of air holes in separator. Although the PI separator had a thin organic film on its surface, it could be observed that this type of film would be not enough to block the separator’s air holes [25,26]. Therefore, it is confirmed that the cyclic degradation was meaningfully attributed to separator pore clogging induced by electrochemical oxidation in interface between electrode and separator during cyclic electrochemical operation.

Comparison of capacity retention of pouch type lithium-ion cells with PE and PI separators at a room temperature (a) and elevated temperature (45℃).
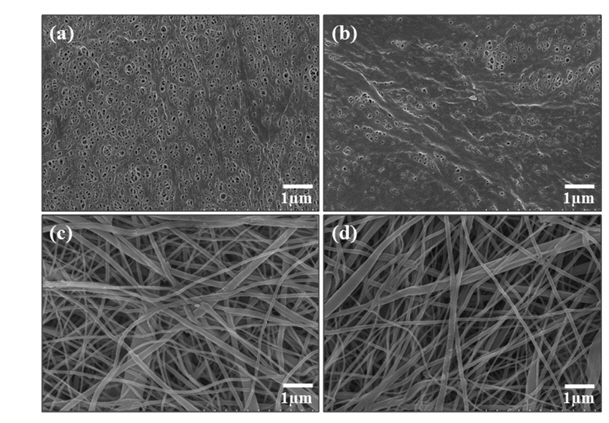
Comparison of separator morphology after 100th charge/discharge cycles at room temperature: (a) PE separator before cycling, (b) PE separator after cycling, (c) PI separator before cycling, and (d) PI separator after cycling.
The high-rate discharge properties for pouch stack cells with one of the separators were evaluated. As shown in Fig. 7, the cell applying PI separator with higher air porosity showed relatively better high-rate properties. In general, at extremely high C-rate (i.e. 10 C, etc.), concentration polarization associated with the ion conductivity inside an electrolyte dominates over an activation polarization that is triggered by the interface resistance between the active material and the electrolyte. However, the difference in discharge capacity of two kinds of separators is not noticeable even at 10 C rate as shown in Fig. 7. It is likely thought that the electrode in the cells is thin enough to show the difference of mobility of lithium ion by the separator.
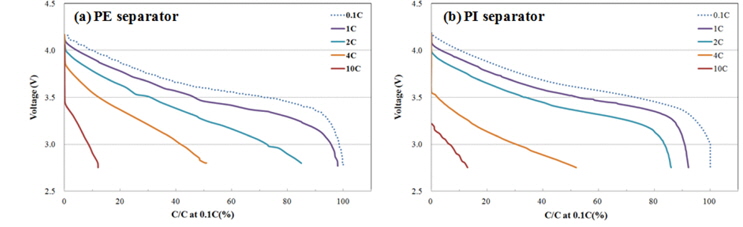
Rate capability of pouch type lithium-ion cell with PE (a) and PI (b) separators. Cells were discharged at various C-rates (0.1, 1, 2, 4 and 10 C) after CC-CV charging at 0.2 C to 4.2 V at room temperature.
A full cell for each separator was made with graphite / LNCMO and tested for trickling charging, which is a way of charging a battery at constant voltage (i.e. 4.3 V and 4.5 V) until the current reaches zero. In addition, SEM/EDS analysis was conducted to check for any metal dissolution on a fully-charged anode’s surface or any metal dissolution on a corresponding cathode’s surface. This test is used to prove that a PI separator’s air hole size can be regarded as a positive dynamical property like high-rate and low-temperature properties, but is closely related to the movement of undesired metal ions triggered by the shuttle mechanism. As shown in the Fig. 8 after continuous charging at 4.2 V for hours, the PE based cell had no metal complex deposition on the interface between anode and separator, while the PI based one had a trace of metal complex deposition, especially for such metals as Mn, Co and Ni. A similar phenomenon occurred when the battery life tests were repeated at room temperature and high temperature. Metal ion elution phenomenon triggered by the oxidation reaction between a fully-charged anode’s surface and the electrolyte surface cannot be avoided, and this phenomenon becomes more apparent at high temperature and high-voltage conditions. Therefore, the PI separator normally goes through a more influential metal ion transition phenomenon, and this kind of phenomenon becomes more serious at certain conditions.
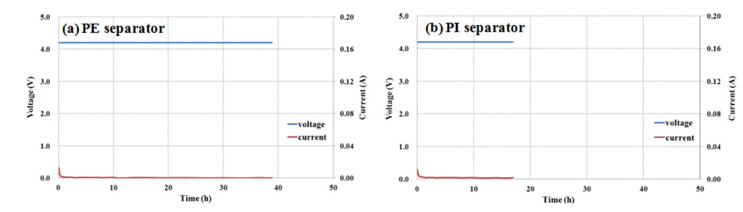
Current profiles during continuous charging at 4.2 V to for pouch type lithium-ion cell with (a) PE and (b) PI separators.
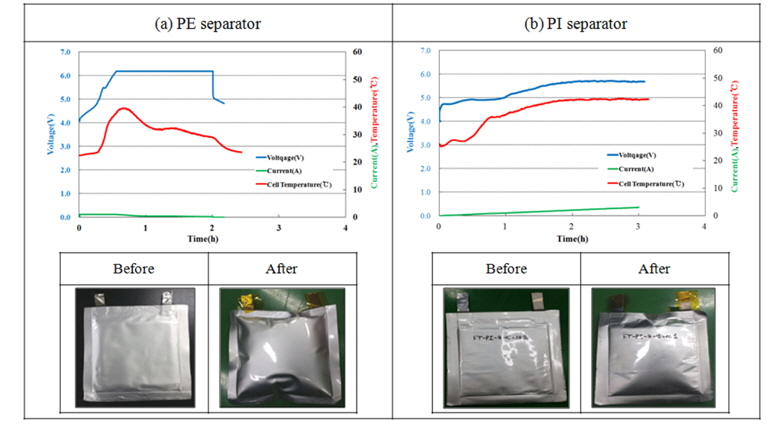
Nail penetration test is to observe the effect of the heat occurring inside the battery by constructing electronic short-circuit in the cell as penetrating metal nail into the center of full cell at a steady speed. A nail penetration test was taken to penetrate metal nail into the fully charged Full cell at a speed of 8 cm·sec−1 in 3.0 mm of diameter. The surface temperature of the cell is as in the Fig. 9. The existing separation film was maintained after the test without the changes of temperature and voltage, however, PI super fine fiber separation film showed the voltage decreased, but it was not connected to the serious heat or explosion after penetration, but it is needed to examine the form after the revival nail penetration test or cell disassembly.
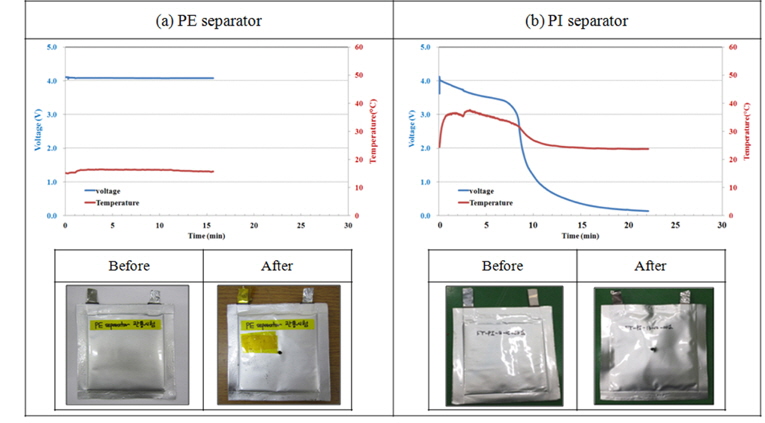
Test results of nail penetration for lithium-ion cell with (a) PE and (PI) separator after full charging.
Overcharge test was taken for the fully charged 1,000 mAh battery to 4.2 V under the condition to check the current rate charging of 1 C to 6.2 V (CC-CV) for 2.5 hours. The features of overcharging according to the separator are described in the Fig. 10 with current density, voltage behavior, and the temperature changes on the surface of cell. The voltage reached to 6.2 V in the PE cell, but only about 5.8 V in the PI cell after applying 6.2 V overcharge test. However, both cells show low temperature spike and current, as well as no ignition or explosion after overcharging.
4. Conclusion
The overall evaluation result indicates that the cell with PI separator showed superior performance over the PE separators one. Cell performance, electrochemical stability, and thermal stability properties for both PE and PI separators were extensively compared and analyzed. Especially, in the hot-box test at 150 and 180℃, the PI separator showed a contraction percentage close to 0% at 150℃, while the PE separator showed a contraction percentage greater than 10% in both width and length. Moreover, at 180℃, the PI separator showed a contraction percentage equal to only 0%, while the PE separator showed a contraction percentage greater than 25%. The PI separators are also evaluated to have higher capacity, higher rate capability compared to the PE separator, indicating that they are ideal candidates for separator of LIBs to achieve high battery performance.
Acknowledgements
This work was supported by the Korea Evaluation Institute of Industrial Technology funded by the Ministry of Knowledge Economy (MKE-2012-10040033) and the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2011-C1AAA001-0030538).
References
F. Croce, G.B. Appetecchi, L. Persi, and B. Scrosati, Nature, 394, 456 (1998).
Croce F., Appetecchi G.B., Persi L., Scrosati B.. Nature 1998;394:456. 10.1038/28818.G. Venugopal, J. Moore, J. Howard, and S. Pendalwar, J. Power Sources, 77, 34 (1999).
Venugopal G., Moore J., Howard J., Pendalwar S.. J. Power Sources 1999;77:34. 10.1016/S0378-7753(98)00168-2.B.L. Luan, G. Campbell, M. Gauthier, X.Y. Liu, I. Davidson, J. Nagata, M. Lepinay, F. Bernier, S. Argue, ECS Transactions, 25, 59 (2010).
Luan B.L., Campbell G., Gauthier M., Liu X.Y., Davidson I., Nagata J., Lepinay M., Bernier F., Argue S.. ECS Transactions 2010;25:59.R.J. Brodd, H.M. Friend, J.C. Nardi, Lithium Ion Battery Technology, ITE-JEC Press, 1995.
Brodd R.J., Friend H.M., Nardi J.C.. Lithium Ion Battery Technology ITE-JEC Press; 1995.U. von Sacken, E. Nodwell, A. Sundher, and J.R. Dahn, Solid State Ionics, 69, 284 (1994).
von Sacken U., Nodwell E., Sundher A., Dahn J.R.. Solid State Ionics 1994;69:284. 10.1016/0167-2738(94)90417-0.U. von Sacken, E. Nodwell, A. Sundher, and J. Dahn, Solid State Ionics, 69, 284 (1994).
von Sacken U., Nodwell E., Sundher A., Dahn J.. Solid State Ionics 1994;69:284. 10.1016/0167-2738(94)90417-0.S. Tobishima and J. Yamaki, J. Power Sources, 81, 882 (1999).
Tobishima S., Yamaki J.. J. Power Sources 1999;81:882.J. Yamaki, in: M. Wakihara, O. Yamamoto (Eds.), Lithium Ion Batteries, 83, Kodansha and Wiley-VCH, Tokyo, Japan (1998).
Yamaki J.. In : Wakihara M., Yamamoto O., eds. Lithium Ion Batteries Kodansha and Wiley-VCH. Tokyo, Japan: 1998. p. 83.J. Cho, Y. Kim, T. Kim, and B. Park, Chem. Mater., 13, 18 (2001).
Cho J., Kim Y., Kim T., Park B.. Chem. Mater. 2001;13:18. 10.1021/cm000759+.H. Kweon, S. Kim, and D. Park, J. Power Sources, 88, 255 (2000).
Kweon H., Kim S., Park D.. J. Power Sources 2000;88:255. 10.1016/S0378-7753(00)00368-2.R. Leising, M. Palazzo, E. Takeuchi, and K. Takeuchi, J. Electrochem. Soc., 148, A838 (2001).
Leising R., Palazzo M., Takeuchi E., Takeuchi K.. J. Electrochem. Soc. 2001;148:A838. 10.1149/1.1379740.A. Dey, J. Electrochem. Soc., 118, 1547 (1971).
Dey A.. J. Electrochem. Soc. 1971;118:1547. 10.1149/1.2407783.A. Christie, S. Lilley, E. Staunton, Y. Andreev, and P. Bruce, Nature, 433, 50 (2005).
Christie A., Lilley S., Staunton E., Andreev Y., Bruce P.. Nature 2005;433:50. 10.1038/nature03186.A. M. Stephan, Europ. Polymer J., 42, 21 (2006).
Stephan A. M.. Europ. Polymer J. 2006;42:21. 10.1016/j.eurpolymj.2005.09.017.K. Morigaki, N. Kabuto, and K. Haraguchi, Matsushita Electric Industrial, US Patent 5597659, issued Jan. 28, 1997.
Morigaki K., Kabuto N., Haraguchi K.. US Patent Matsushita Electric Industrial; issued Jan. 28, 1997. 5597659.K. Abraham, M. Alamgir, and D. Hoffman, J. Electrochem. Soc., 142, 683 (1995).
Abraham K., Alamgir M., Hoffman D.. J. Electrochem. Soc. 1995;142:683. 10.1149/1.2048517.D. Kim, K. Noh, J. Chun, S. Kim, and J. Ko, Solid State Ionics, 144, 329 (2001).
Kim D., Noh K., Chun J., Kim S., Ko J.. Solid State Ionics 2001;144:329. 10.1016/S0167-2738(01)00977-8.Y. Wang, J. Travas-Sejdic, and R. Steiner, Solid State Ionics, 148, 443 (2002).
Wang Y., Travas-Sejdic J., Steiner R.. Solid State Ionics 2002;148:443.F. Ooms, E. Kelder, J. Schoonman, N. Gerrits, J. Smedinga, and G. Callis, J. Power Sources, 97, 598 (2001).
Ooms F., Kelder E., Schoonman J., Gerrits N., Smedinga J., Callis G.. J. Power Sources 2001;97:598.Y. Miaoa, G. Zhub, H. Houc, Y. Xiab, and T. Liua, J. Power Sources, 226, 82 (2013).
Miaoa Y., Zhub G., Houc H., Xiab Y., Liua T.. J. Power Sources 2013;226:82. 10.1016/j.jpowsour.2012.10.027.L. Carnell, E. Siochi, N. Holloway, R. Stephens, C Rhim, L. Niklason, and R. Clark, Macromolecules, 5345, 14 (2008).
Carnell L., Siochi E., Holloway N., Stephens R., Rhim C., Niklason L., Clark R.. Macromolecules 2008;5345:14.D. Chen, T. Liu, X. Zhou, W. Tjiu, and H. Hou, J. Phys. Chem., B, 113, 9741 (2009).
Chen D., Liu T., Zhou X., Tjiu W., Hou H.. J. Phys. Chem., B 2009;113:9741.D. Chen, R. Wang, W. Tjiu, and T. Liu, Compos. Sci. Technol., 71, 1556 (2011).
Chen D., Wang R., Tjiu W., Liu T.. Compos. Sci. Technol. 2011;71:1556. 10.1016/j.compscitech.2011.06.013.Y. Kim, H. Kim, C. Doh, S. Kim, and S. Lee. J. Power Sources, 244, 196 (2013).
Kim Y., Kim H., Doh C., Kim S., Lee S.. J. Power Sources 2013;244:196. 10.1016/j.jpowsour.2013.01.166.P. Ma and R. Zhang, J. Biomedical Mater. Resear., 46, 60 (1999).
Ma P., Zhang R.. J. Biomedical Mater. Resear. 1999;46:60. 10.1002/(SICI)1097-4636(199907)46:1<60::AID-JBM7=3.0.CO;2-H.D. Reneker and I. Chun, Nanotechnology, 7, 216 (1996).
Reneker D., Chun I.. Nanotechnology 1996;7:216. 10.1088/0957-4484/7/3/009.Y. Kim, H. Kim, C. Doha, S. Kim, and S. Lee, J. Power Sources, 244, 196 (2013).
Kim Y., Kim H., Doha C., Kim S., Lee S.. J. Power Sources 2013;244:196. 10.1016/j.jpowsour.2013.01.166.