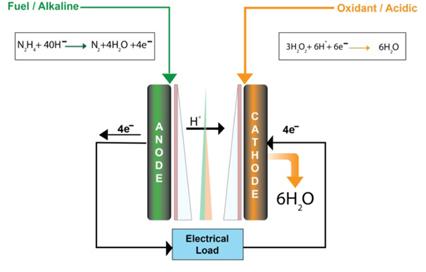
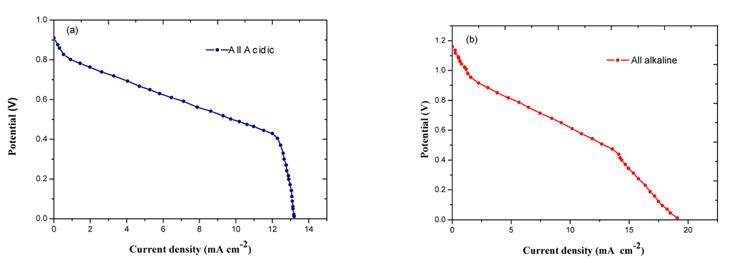
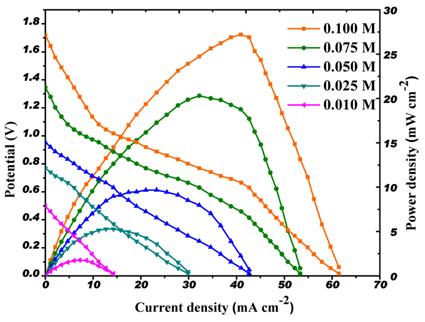
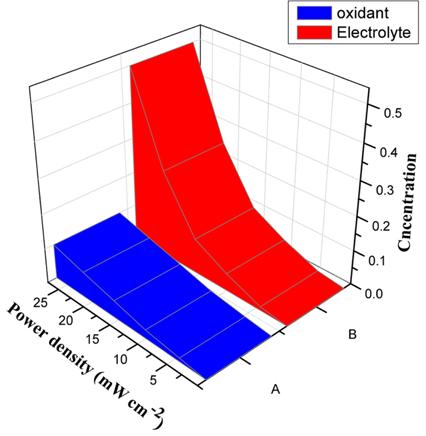
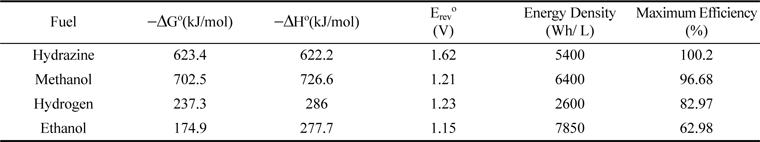
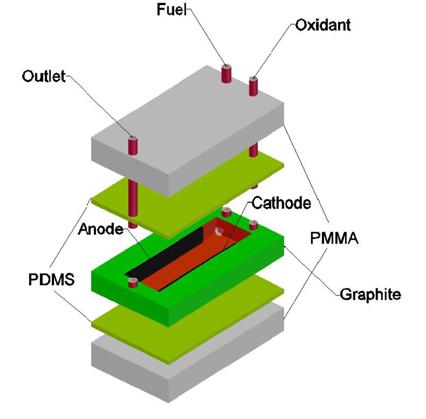
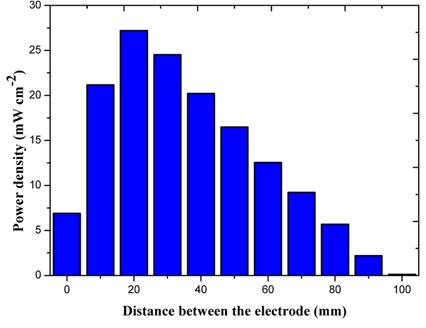
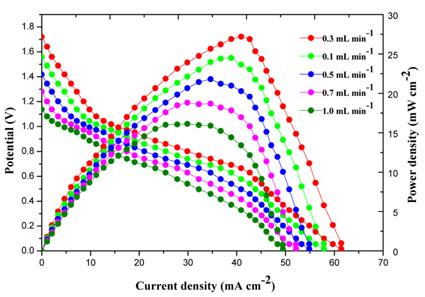
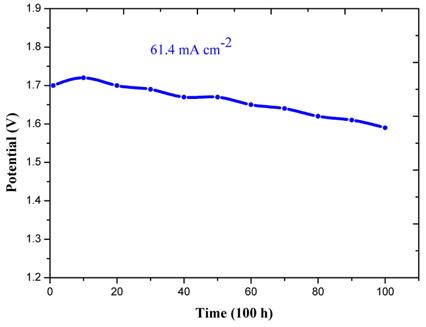
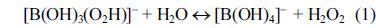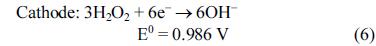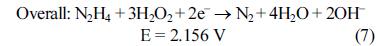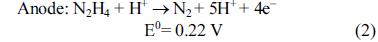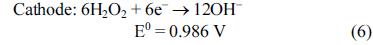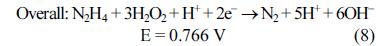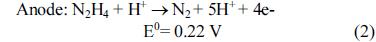[1]
De Jong J.
Lammertink R.G.H.
Wessling M.
Lab on a chip., 2006, 6, 1125.
[2]
Kjeang E.
Djilali N.
Sinton D.
J. Power Sources,
2009,
186, 353.

[3]
Mousavi Shaegh S.A.
Nguyen N.T.
Chan S.H.
Int. J. Hydrogen Energy, 2011, 37, 3466.
[4]
Flipsen S.F.J.
J. Power Sources,
2006,
162, 927.

[5]
Choban E.R.
Markoski L.J.
Wieckowski A.
Kenis P.J.A.
J. Power Sources,
2004,
128, 54.

[6]
Cotton F.A.
Wilkilson G.
Advanced Inorganic Chemistry. Wiley Interscience, New York, 1988.
[7]
Karunakaran C.
Muthukumaran B.
Transition Met Chem.,
1995,
20, 460.


[8]
Gowdhamamoorthi M.
Arun A.
Kiruthika S.
Muthukumaran B.
Int. Journal of ChemTech Research, 2013, 5, 1143.
[9]
Gowdhamamoorthi M.
Arun A.
Kiruthika S.
Muthukumaran B.
Journal of Materials, 2013, 2013, 7.
[10]
Yamada Koji
Asazawa Koichiro
Yasuda Kazuaki
Ioroi Tsutomu
Tanaka Hirohisa
Miyazaki Yoshinori
Kobayashi Tetsuhiko
J. Power Sources, 2003, 115, 236.
[11]
Serov Alexy
Kwak Chan
Applied catalysis B: Environmental., 2010, 98(1), 98.
[12]
Bard A. J.
Anal. Chem.,
1963,
35, 1602.

[13]
Burke L.D.
OŌĆÖDwyer K.J.
Electrochim. Acta,
1989,
34, 1659.

[14]
Arun A.
Gowdhamamoorthi M.
Kiruthika S.
Muthukumaran B.
J. Electrochem. Soc., 2014, 161(3), 1ŌĆō7.
[15]
Kenis P.J.A.
Ismagilov R.F.
Whitesides G.M.
Science, 1999, 83, 285.
[16]
Arun A.
Gowdhamamoorthi M.
Kiruthika S.
Muthukumaran B.
Int. J. ChemTech Research, 2013, 5, 1152.
[17]
Ponmani K.
Durga S.
Gowdhamamoorthi M.
Kiruthika S.
Muthukumaran B.
Ionics,
2014,
20(
11), 1579ŌĆō1589.

[18]
Choban E.R.
Waszczuk P.
Markoski L.J.
Wieckowski A.
Kenis P.J.A.
In: Procedings of the First International Conference on Fuel Cell Science Engineering and Technology; Rochester, NY. 2003.
[19]
Choban E.R.
Markoski L.J.
Stoltzfus J.
Moore J.S.
Kenis P.J.A.
In: Proceedings of the 40th Power Sources Conference; Cherry Hill, NJ. 2002.
[20]
Spendelow J.
Lu G.Q.
Kenis P.J.A.
Wieckowski A.
J. Electroanal. Chem.,
2004,
568, 215.

[21]
McLean G.F.
Niet T.
Prince-Richard S.
Djiali N.
Int. J. Hydrogen Energy,
2002,
27, 507.

[22]
Iwasita T.
Electrochim. Acta,
2002,
47, 3663.

[23]
Lide D.R.
(Ed.)
CRC Handbook of Chemistry and Physics MARC Records Transmitter System. 2004.
[24]
Yu E.H.
Scott K.
J. Power Sources,
2004,
137, 248.

[25]
Choban E.R.
Spendelow J.S.
Gancs L.
Wieckowski A.
Kenis P.J.A.
Electrochim Acta,
2005,
50, 5390.

[26] .
Ponmani K.
Durga S.
Arun A.
Kiruthika S.
Muthukumaran B.
Int. J. Electrochemistry, 2014.
[27]
Ismagilov R.F.
Stroock A.D.
Kenis P.J.A.
Whitesides G.
Appl Phys Lett,
2000,
76, 2376.

[28]
Chang M.
Chen F.
Fang N.
J. Power Sources,
2006,
159, 810.

[29]
Sung W.
Choi J.W.
J. Power Sources,
2007,
172, 198.

[30]
Bazylak A.
Sinton D.
Djilali N.
J. Power Sources,
2004,
143, 57.

[31]
Kjeang E.
McKechnie J.
Sinton D.
Djilali N.
J. Power Sournces,
2007,
168, 379.