Electrodeposition of Mn-Ni Oxide/PEDOT and Mn-Ni-Ru Oxide/PEDOT Films on Carbon Paper for Electro-osmotic Pump Electrode
Article information
Abstract
MnO2, a metal oxide used as an electrode material in electrochemical capacitors (EDLCs), has been applied in binary oxide and conducting polymer hybrid electrodes to increase their stability and capacitance. We developed a method for electrodepositing Mn-Ni oxide/PANI, Mn-Ni oxide/PEDOT, and Mn-Ni-Ru oxide/PEDOT films on carbon paper in a single step using a mixed bath. Mn-Ni oxide/PEDOT and Mn-Ni-Ru oxide/PEDOT electrodes used in an electro-osmotic pump (EOP) have shown better efficiency compared to Mn-Ni oxide and Mn-Ni oxide/PANI electrodes through testing in water as a pumping solution. EOP using a Mn-Ni-Ru oxide/PEDOT electrode was also tested in a 0.5 mM Li2SO4 solution as a pumping solution to confirm the effect of the Li+ insertion/de-insertion reaction of Ruthenium oxide on the EOP. Experimental results show that the flow rate increases with the increase in current in a 0.5 mM Li2SO4 solution compared to that obtained when water was used as a pumping solution.
1. Introduction
In a traditional electro-osmotic pump (EOP), Pt is mainly used as an electrode. However, the high price of Pt when used as an electrode increases the price of the EOP; moreover, EOP also requires a high driving voltage. The use of Pt electrodes and a high voltage for EOP operation also incurs a problem in generating O2 and H2 through the electrolysis of water. To solve this problem, an additional device is required to remove the generated gas, although there is no need to increase the size of the EOP itself. However, because of the use of an additional device, the size of all equipment required including the EOP itself becomes inevitably large. To solve these problems caused through the use of a Pt electrode, we focused on the reactions occurring in the electrode and reported an EOP using electrodes of Ag/Ag2O and PANI-PSS [1]. An EOP using the above materials as an electrode does not generate any gas owing to the electrolysis of water and can be powered by a battery because it can be driven at a low voltage.
The materials used as electrodes for electric double-layer capacitors (EDLCs), also known as a supercapacitor or ultracapacitor, undergo a redox reaction for energy storage, and they can be applied as an electrode material for an EOP. EDLCs have received significant interest owing to a higher power density and longer cycle life than those of a conventional battery [2-4]. The capacitance of an EDLC can be increased by utilizing pseudo-capacitive materials, mainly transition metal oxides such as ruthenium oxide (RuO2), iron oxide (Fe3O4), and manganese oxide (MnO2), owing to their multiple valence states and electroactive conducting polymers (ECPs), such as polypyrroles, polyanilines, and polythiophenes [5-7]. The charge storage mechanism of these materials relies on rapid and reversible faradaic surface reactions, and offers a means of achieving high density at a high charge–discharge rate. In general, metal oxides have a high specific capacitance, but some metal oxides (MnO2, Co3O4, CoO, NiO, and V2O5) exhibit poor electrical conductivity. In addition, metal oxides generally have poor ion transport kinetics. Efforts are being made to address this problem [8].
Conductive polymer is cheaper than metal oxide, and has certain advantages such as a high charge density, good intrinsic conductivity, fast charge–discharge rates, and easy synthesis through electropolymerization [9]. However, a relatively low capacitance and relatively low cycling stability arise from the swelling and contraction caused by the insertion and removal of ions and from the polymer applied during the charge-discharge cycles [10]. In order to overcome the problems arising from the use of metal oxides and ECPs, many research groups have been making efforts to improve the performance of EDLC electrodes by developing a hybrid electrode using a mixture of metal oxides and ECPs [11-15].
MnO2 has attracted significant attention as an electrode material for use in electrochemical capacitors, which can replace RuO2, owing to such advantages as a satisfactory electrochemical performance, relatively low cost, and natural abundance [16, 17]. In addition, electrodes have been reported in which binary metal oxides such as manganese-nickel oxide and manganese-cobalt oxide are mixed together to improve the specific capacitance and make the electrode more stable than when using MnO2 alone [18-21]. PANI is one of the most promising conductive polymers owing to its low cost, easy and economic synthesis, controllable electric conductivity, high electroactivity, and good environmental stability [22, 23]. PEDOT is water insoluble and highly conductive (300 S/cm) with a very high stability in an oxidized state. It is usually used as a PEDOT-PSS film by applying poly(styrenesulfonate) as an anionic material to balance the charge and improve its water-insoluble properties. PEDOT-PSS has good conductivity (10 S/cm), high visible-light transmittance, and very good stability at 1000°C for over 1,000 h [24].
To improve the performance of the previously reported Mn-Ni oxide film, we developed Mn-Ni oxide/PANI, Mn-Ni oxide/PEDOT, and Mn-Ni-Ru oxide/PEDOT films through a one-step electrodeposition on carbon paper. All components can be easily produced through electrochemical oxidation under similar potentials, which makes it possible to easily create a hybrid surface. A detailed discussion of the electrochemical studies of each electrode created, and performance results of EOPs made using the new electrodes, are discussed.
2. Experimental
2.1. Chemicals
The chemicals used in this study are as follows: Mn(CH3COO)2·4H2O (Sigma-Aldrich), NiCl2·6H2O (Sigma-Aldrich), RuCl3·xH2O (Sigma-Aldrich), aniline (Sigma-Aldrich), 3,4-ethylenedioxythiophene (EDOT, TCI), HCl (Sigma-Aldrich), KCl (Jin Chemical), Na2SO4 (Jin Chemical), and Li2SO4 (Alfar-Aesar). All chemicals were of analytical reagent grade and directly used without further purification.
2.2. Electrode fabrication and electrochemical study
All films were created using an electroplating method. A mixture of 0.1 M Mn(CH3COO)2·4H2O and 0.2 M NiCl2·6H2O was used for the Mn-Ni oxide films, a mixture of 0.1 M Mn(CH3COO)2·4H2O, 0.2 M NiCl2·6H2O, and 0.01 M aniline was used for the Mn-Ni oxide/PANI films, and a mixture of 0.1 M Mn(CH3COO)2·4H2O, 0.2 M NiCl2·6H2O, and 0.01 M EDOT was used for the Mn-Ni oxide/PEDOT films. Mn-Ni-Ru oxide/PEDOT films on carbon paper were also electrodeposited in a mixture of 0.1 M Mn(CH3COO)2·4H2O, 0.2 M NiCl2·6H2O, 0.02 M RuCl3·xH2O, 0.01 M EDOT, and 0.1 M KCl in 0.01 M HCl. Carbon paper pre-treated with air plasma for 10 min was applied both as the working and auxiliary electrodes. The mixture solution was electro-oxidized using carbon paper at 1.2 V for 30 min. After electroplating, the electrode was washed with water and heated at 150 °C for 1 h. Finally, the electrode was sonicated in water and vacuum dried.
Electroplating and cyclic voltammetry were conducted using CHI 440A and CHI 600C (CH Instruments). A one-pot cell was used for all experiments. Auxiliary electrode was carbon papers for electrodeposition and DSA electrodes for CV. A Ag/AgCl (3 M KCl) electrode was used as a reference electrode.
2.3. EOP assemble and testing
The EOP was configured as shown in Fig. 1. A single silica membrane was connected symmetrically with each of the electrodes, Ag contact strips, and PC frames, and finally sealed with epoxy bonding. The EOP was fully soaked in a pumping solution to be used before measuring the performance. The EOP was controlled and monitored using an EOPump controller (KS RnD), and was tested by applying 2.5 V for 30 s, followed by −2.5 V for 30 s, as a single cycle.
3. Results and Discussion
3.1. Electrochemical study of Mn-Ni oxide, Mn-Ni oxide/PANI, and Mn-Ni oxide/PEDOT
The electrode fabrication methods are described in detail in the experimental section. Carbon paper was treated with air plasma for 10 min prior to electrodeposition. It was previously reported that treating carbon fiber or carbon paper surfaces with air plasma results in an increase in oxygen content [25]. Various functional groups make the carbon surface more polar, and a carbon surface can easily react with the material for electrodeposition and increase the surface adhesion. We first fabricated the surface of Mn-Ni oxide, which was created for comparison, and then fabricated a hybrid Mn-Ni oxide/conducting polymer surface using a conducting polymer. Aniline and EDOT were used as precursors to create the conducting polymer. The prepared electrode was annealed at 150 °C for 1 h to stabilize the surface. After annealing, sonication was conducted in water. Sonication was applied to remove the films adhering weakly to the surface. Finally, vacuum drying was conducted prior to use.
Fig. 2 shows the cycle voltammogram of each electrode prepared in a 0.5 M Na2SO4 solution. The peak current continuously increased in both the positive and negative scan directions in all CVs, indicating that oxidation and reduction reactions continuously occurred. This continuous reaction cannot stably maintain the electrode surface, and when used for a long period of time, the performance of the electrode is expected to deteriorate owing to the change in the surface. Table 1 shows the electrode capacitance calculated at a scan rate of 10 mV/s based on a CV measured in a 0.5 M Na2SO4 solution. It shows almost the same value for all electrodes. Thus, it seems that the added conductivity polymer has little influence on the change of the capacitance.
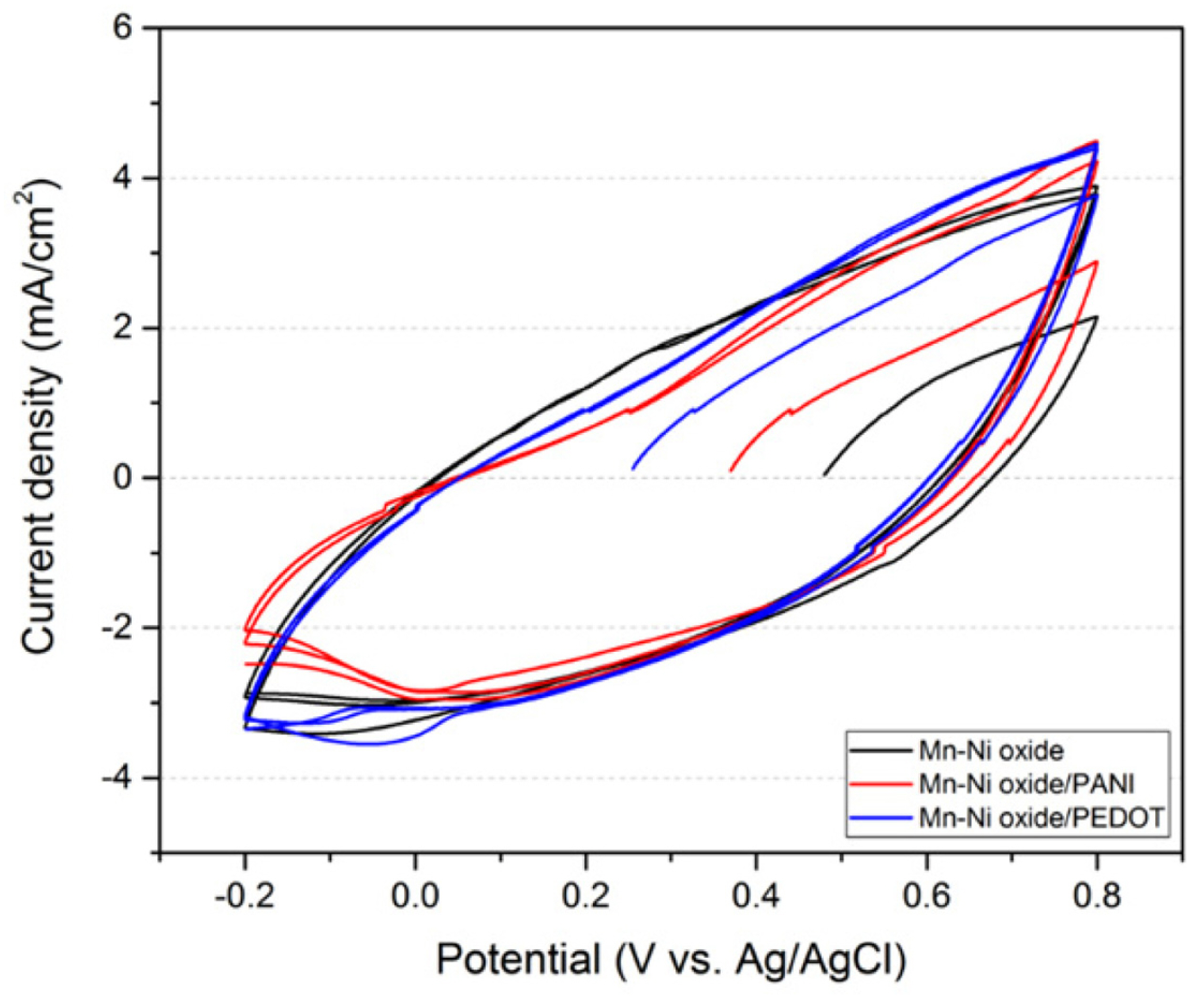
Cyclic voltammogram of Mn-Ni oxide, Mn-Ni oxide/PANI, and Mn-Ni oxide/PEDOT films on carbon paper in a 0.5 M Na2SO4 solution at a scan rate of 10 mV/s.
3.2. Mn-Ni oxide, Mn-Ni oxide/PANI, and Mn-Ni oxide/ PEDOT electrodes used in EOP test
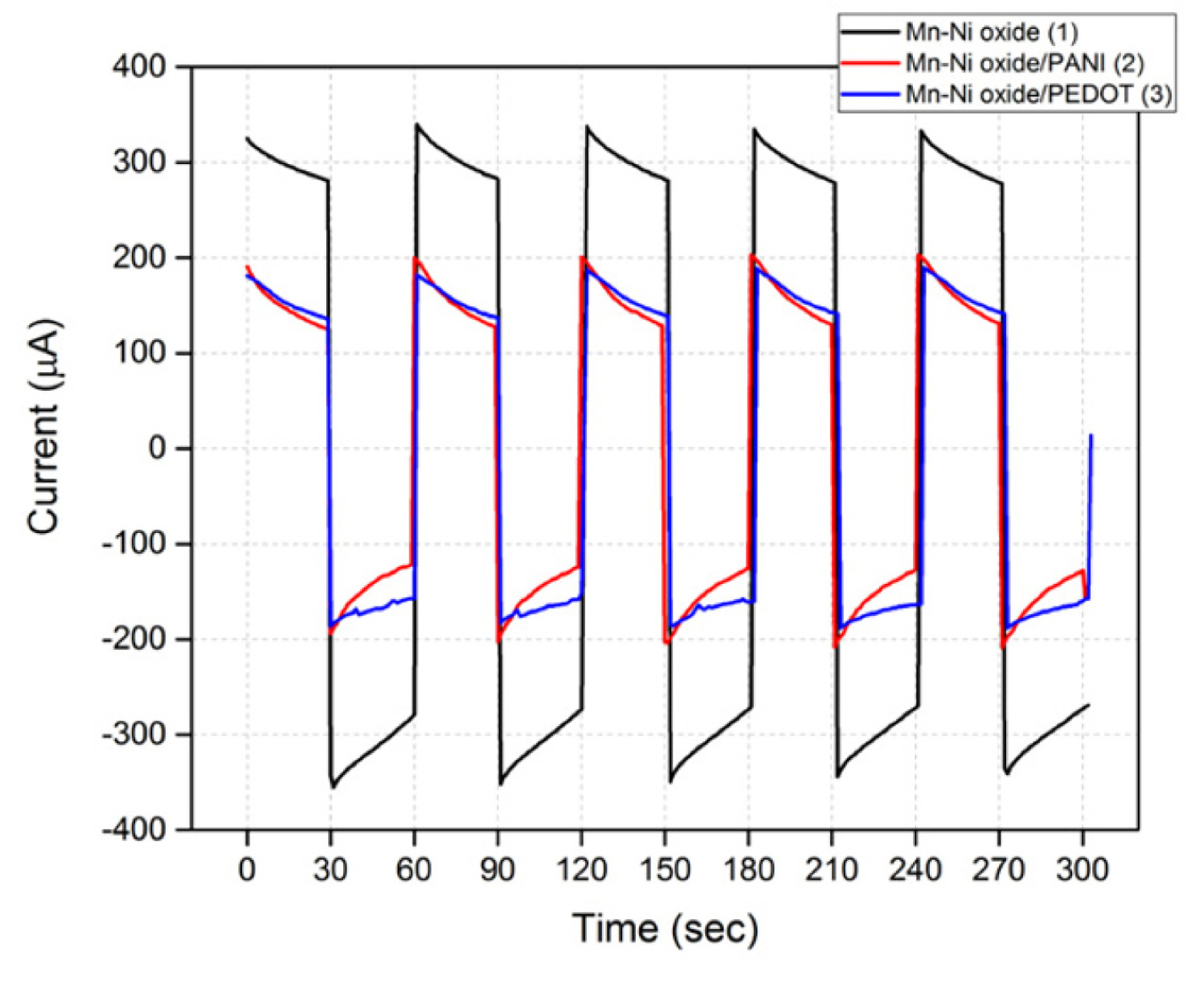
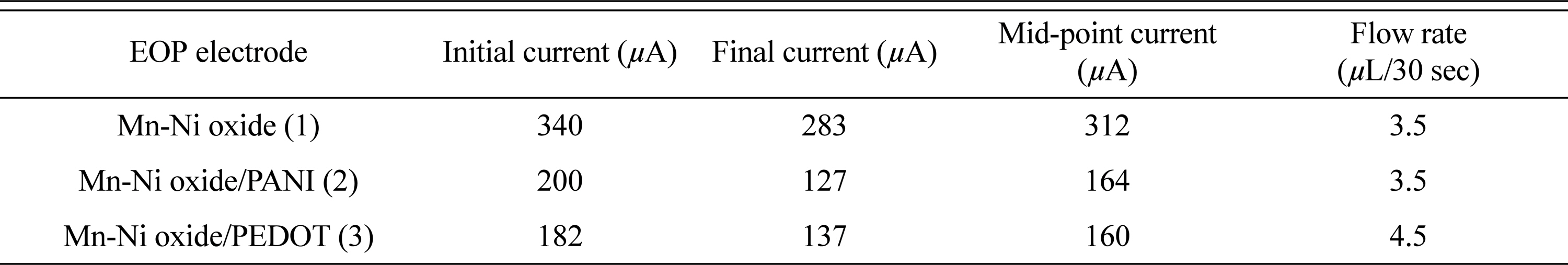
Mn-Ni oxide (1), Mn-Ni oxide/PANI (2), and Mn-Ni oxide/PEDOT (3) was made of EOP, as shown in Fig. 1. The prepared EOP was sufficiently wetted with water for use as a pumping solution, and all gas bubbles in the EOP were removed and used. Table 2 shows the current response and flow rate of the EOPs using each electrode. For EOP testing, 1 cycle of 2.5 V for 30 seconds and −2.5 V for 30 seconds were measured for 5 cycles. In the case of an EOP using the type (1) electrodes, the initial and final currents were the highest, but the flow rate was the lowest. However, the current of the EOP using type (2) and (3) electrodes, which added a conducting polymer to type (1), was significantly lower than the current when applying type (1). Comparing types (2) and (3), type (3) shows a high flow rate with a low current, and thus can be judged as a more effective electrode for an EOP. Comparing types (1) and (3), the current is reduced by 49%, and the flow rate is increased by 29%, indicating that the addition of PEDOT to Mn-Ni oxide makes it possible to produce an EOP-efficient electrode. Fig. 3 also shows that the changes in the initial and final currents of an EOP using a type (3) electrode are the smallest.
Current variations and flow rates of Mn-Ni oxide, Mn-Ni oxide/PANI, and Mn-Ni oxide/PEDOT electrode used in EOPs in water as a pumping solution.
3.3. Electrochemical study of Mn-Ni-Ru oxide/PEDOT and application for EOP electrode
We determined through past experiments that Mn-Ni oxide/PEDOT is a proper electrode for an EOP, and decided to add Ruthenium oxide to improve the electrode performance. Ruthenium oxide can be produced from RuCl3 at a similar potential at which Mn-Ni oxide/PEDOT is electrodeposited. In Fig. 4, the CV of the fabricated Mn-Ni-Ru oxide/PEDOT is compared. The calculated capacitance was 328 mC/cm2 for the Mn-Ni-Ru oxide/PEDOT, and slightly increased from 300 mC/cm2 for the Mn-Ni oxide/PEDOT. In addition, it can be predicted that the electrode can be stably maintained because the change in current of Mn-Ni-Ru oxide/PEDOT is smaller than that of Mn-Ni oxide/PEDOT from the CV.

Cyclic voltammogram of Mn-Ni oxide/PEDOT and Mn-Ni-Ru oxide/PEDOT films on carbon paper in 0.5 M Na2SO4 solution at a scan rate of 10 mV/s.
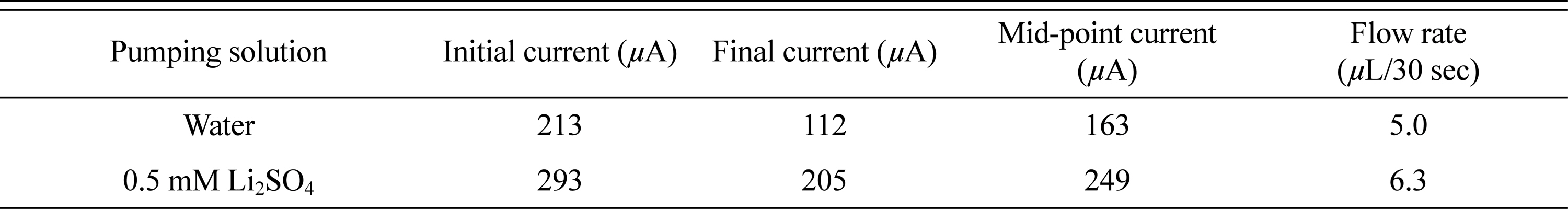
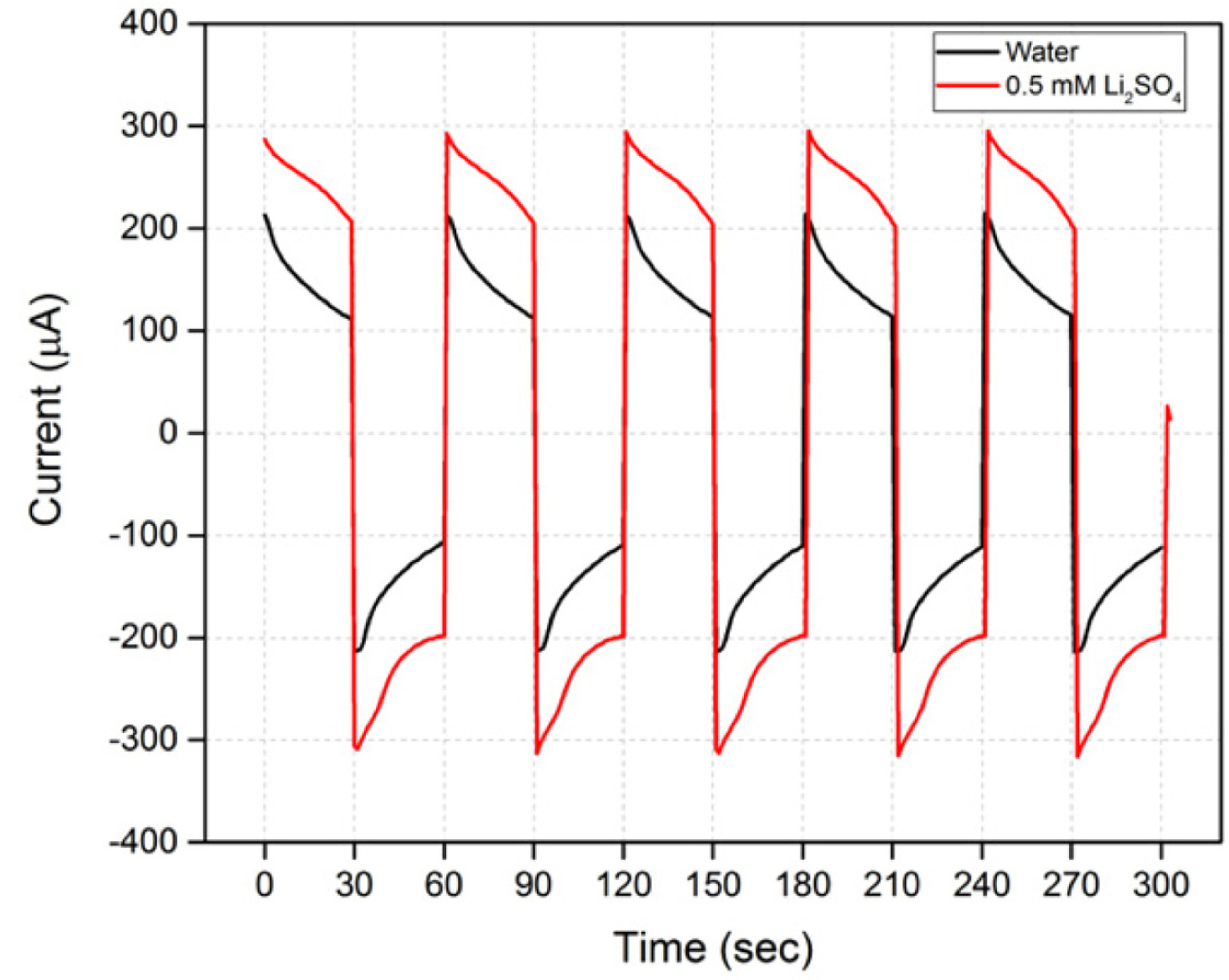
EOPs fabricated using Mn-Ni-Ru oxide/PEDOT (4) electrodes were tested in two pumping solutions. In the case of Ruthenium oxide, it is known that additional capacitance can be realized through the insertion/de-insertion reaction of Li+, in addition to the energy storage mechanism using H+ [26-30]. Therefore, a comparative experiment of the pumping solution water and a 0.5 mM Li2SO4 solution will be able to determine the effects of the interaction between Ruthenium oxide and Li+ on the performance of an EOP. When water was used as a pumping solution, the type (4) electrode used in an EOP showed a flow rate of 5 μA/30 s with a median current of 163 μA. Compared with a type (3) electrode, the median current is very similar, but the difference between the initial and final currents is slightly increased, as is the flow rate.
Table 3 shows the difference in EOP performance when used in water and 0.5 mM Li2SO4 as a pumping solution for a type (4) electrode. The current is increased by 80 μA (38%) compared with water, and the flow rate is also increased by 1.3 μA/30 s (26%) when 0.5 mM Li2SO4 is used. Although the rate of increase in the flow rate cannot follow the rate of increase of the current, we know that a high flow rate can be realized by changing the Li2SO4 solution into a pumping solution in an EOP when using a type (4) electrode. Fig. 5 shows that the slope of the current change remains similar, despite an increase in current owing to a change in the pumping solution. Changes in the performance of an EOP using type (4) electrodes with changes in the concentration of the Li2SO4 solution seem to require further investigation.
4. Conclusions
We developed a simple process to fabricate mixed-metal-oxide/conducting-polymer hybrid electrodes using a single electrodeposition. The CVs of Mn-Ni oxide, Mn-Ni oxide/PANI, and Mn-Ni oxide/PEDOT in 0.5 M of Na2SO4 were similar in shape and calculated capacitance; however, an EOP with Mn-Ni oxide/PEDOT electrodes showed the best performance in water. Because Ru oxide can be formed at a similar potential where Mn-Ni oxide/PEDOT is electrodeposited, a Mn-Ni-Ru oxide/PEDOT surface was fabricated using one-step electrodeposition. The CV shape of Mn-Ni-Ru oxide/PEDOT is more rectangular than that of Mn-Ni-Ru oxide/PEDOT, and their capacitance is calculated as 328 and 300 mC/cm2 , respectively. An EOP using a Mn-Ni-Ru oxide/PEDOT electrode was tested in both water and a 0.5 mM Li2SO4 solution as a pumping solution to confirm the effect of the Li+ insertion/de-insertion reaction of Ru oxide. Finally, we confirmed that the flow rate increases with an increase in current in 0.5 mM Li2SO4 as compared to water. Further study is needed to test the longterm use of Mn-Ni oxide/PEDOT and Mn-Ni-Ru oxide/PEDOT electrodes used in an EOP. Experiments will also be conducted to determine whether the performance of an EOP using Mn-Ni-Ru oxide/PEDOT electrodes can be tuned by changing the concentration of Li2SO4.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number, HI13C2197).