|
 |
- Search
| J. Electrochem. Sci. Technol > Volume 15(2); 2024 > Article |
|
Abstract
Due to its high theoretical capacity, Silicon (Si) has shown great potential as an anode material for lithium-ion batteries (LIBs). However, the large volume change of Si during cycling leads to poor cycling stability and low Coulombic efficiency. In this study, we synthesized Si/Carbon C45:Graphene composites using a ball-milling method with a fixed Si content (20%) and investigated the influence of the C45/Gr ratio on the electrochemical performance of the composites. The results showed that carbon C45 networks can provide good conductivity, but tend to break at Si locations, resulting in poor conductivity. However, the addition of graphene helps to reconnect the broken C45 networks, improving the conductivity of the composite. Moreover, the C45 can also act as a protective coating around Si particles, reducing the volume expansion of Si during charging/discharging cycles. The Si/C45:Gr (70:10 wt%) composite exhibits improved electrochemical performance with high capacity (~1660 mAh g−1 at 0.1 C) and cycling stability (~1370 mAh g−1 after 100 cycles). This work highlights the effective role of carbon C45 and graphene in Si/C composites for enhancing the performance of Si-based anode materials for LIBs.
Rechargeable lithium-ion batteries (LIBs) with their high energy density, high power density, long lifespan, and eco-friendliness make them the preferred technology for portable electronics, power tools, and hybrid/full electric vehicles (EVs), when compared to other commonly used batteries [1–4]. Sulfur and oxygen-based materials have been extensively investigated for their potential as cathode materials, while silicon (Si), tin, and lithium (Li) has been predominantly studied for their potential as anode materials in next-generation LIBS [5–7]. Out of these materials, silicon (Si) stands out as a promising option due to its abundance, affordability, nontoxicity, and most importantly, its significantly higher theoretical capacity compared to conventional graphite anodes [8–10]. Despite their high lithium storage capacity, the implementation of silicon anodes in commercial cells is challenging. This is because Si undergoes a large volume change during cycling, the low conductivity of silicon, combined with the material expansion, can lead to reduced Coulombic efficiency (CE), cell fade, and safety concerns, limiting the application of silicon-based materials in lithiumion batteries [11]. To overcome the challenges associated with silicon anodes, Various methods have been explored for the production of silicon-carbon composites, with different advantages and limitations.
One approach is the physical mixing of silicon nanoparticles and graphene, which can be a low-cost and scalable method for the industrial production of electrodes [12–20]. Liu et al. [21] synthesized Si/C bean-structured materials via a simple ball-milling process, and the results demonstrated excellent electrochemical performance, with high specific capacity (1162.2 mAh g−1 at 0.1 C and 902.6 mAh g−1 at 0.2 C) and superior cycling stability (734 mAh g−1) after 200 cycles. However, this method may not always result in optimal electrochemical performance in cells.
Alternative methodologies have been developed to address the limitations of simple physical mixing. Yang et al. [22] have found that a composite of silicon and graphite synthesized through thermal pyrolysis of polyvinyl chloride dispersed with nanosized silicon and fine graphite particles displays a reversible capacity of approximately 700 mAh g−1 with improved cyclability compared to raw nanocrystalline Si. Additionally, ultrasonication combined with thermal treatment in a reducing atmosphere is another approach that has been explored for synthesizing silicon nanoparticles/graphene composites. The resulting silicon-graphene composite can exhibit high specific capacity, with values exceeding 2000 mAh g−1, in half-cell configurations. The composite is also capable of sustaining high currents, with capacities over 750 mAh g−1 at a current rate of 7 A g−1, while maintaining high Coulombic efficiency (CE) of approximately 99% [23]. These alternative methods are more effective in optimizing the electrochemical performance of silicon-graphene composites. However, their synthesis process is complex and difficult to control for uniformity, thus they may have downsides in terms of scalability and compatibility with large-scale industrial production.
Zhang et al. have developed a Si@Graphene material that was synthesized using magnesium reduction of silicon dioxide, resulting in the formation of silicon onto which graphene was deposited using chemical vapor deposition (CVD). The binding between the silicon and graphene establishes a robust and efficient contact, enabling fast electron and ion transport to and from the silicon. The results have demonstrated a stable high-capacity performance, with an approximate capacity of 1500 mAh g−1 at a current rate of 2 A g−1 after 500 cycles [24]. Furthermore, Han et al. have synthesized Si/C particles anchored on a graphene sheet using a cost-effective and environmentally friendly process. They first coated the graphene oxide surface with a polydopamine layer through the self-polymerization of dopamine. Then, silicon particles were firmly anchored onto the graphene sheets. After carbonization, the structure was maintained to prevent aggregation of silicon particles. The resulting anode materials exhibited a high reversible capacity of 1910.5 mAh g−1 and 1196.1 mAh g−1 after 700 cycles at a current density of 357.9 mA g−1 [25]. Despite showing a high electrochemical performance, both methods mentioned above have the drawback of difficulty in achieving high uniformity.
Based on our current knowledge, no publication has proposed the fabrication of Si/C45-Gr composite materials via ball-milling and investigated the effect of the ratio of each component on the stability and specific capacity of the LIBs. In this study, we have synthesized the Si/C45:Gr material using a ball-milling method, with a fixed weight ratio of Si at 20% to investigate the effect of the C45/Gr ratio on the specific capacity of the battery. Carbon C45 exhibits good conductivity, however, when it is used to create a composite with Si, it tends to break the conductivity at the location of Si, resulting in a break in the circuit. Therefore, graphene sheets are added to connect the carbon C45 regions together, improving the conductivity of these materials. Furthermore, the carbon C45 covers the Si particles to reduce the volume expansion of Si.
Silicon nanoparticles (Si, > 99%, 80–100 nm) was procured from SkySpring Nanomaterials, Inc. Graphene (Gr, 0.5–5 μm), lithium hexafluorophosphate (LiPF6, ≥ 99.99%), ethylene carbonate (EC, ≥ 99%), and diethyl carbonate (DEC, anhydrous, ≥ 99%) were provided by Sigma-Aldrich. Super C45 conductive carbon black (C45), super P conductive carbon black (Super P), and carboxymethyl cellulose (CMC, ≥ 99.5%) were supplied by MTI corporation.
The mechanical ball-milling method using an omnidirectional planetary ball-milling mode QXQM-2 from TENCAN POWDER was used to synthesize the composites. The Si nanoparticles, super C45 conductive carbon black (C45), and Graphene (Gr) were combined in the zirconia ball milling tank following the wt% specified in Table 1, and no additional additives were introduced. The weight ratio of the zirconia ball to the Si/Gr/C45 mixture was 10:1 and the rotational speed was set to 500 rpm. After 10 hours of ball-milling, Si/C45:Gr nanocomposites with uniform characteristics were produced.
The crystalline structure of the Si/C45:Gr composites was examined using X-ray diffraction (XRD) with a D8-ADVANCED (Brucker). The nanoparticle’s morphology and elemental structure were observed using Scanning electron microscopy (SEM) with a SU 8010 (Hitachi, Japan) model, and energy-dispersive X-ray spectroscopy (EDS) was performed simultaneously to analyze the distribution of the elements. Further, the detailed morphology structure was investigated via Transmission electron microscopy (TEM) with a JEM-4010 (JEOL) instrument.
The electrochemical properties of the Si/C45:Gr composites were evaluated using a 2023-coin-type half-cell assembled in an argon-filled glove box. The working electrodes were prepared by mixing the active materials, carbon super P, and carboxymethyl cellulose (CMC) in a weight ratio of 8:1:1. The resulting mixture was stirred for about an hour using a blender to create a homogeneous slurry, and then coated to Cu foil used as the current collector. The observed electrode had an electrode density is nearly 3.0 mg cm−2 and a loading level of 0.48 mg cm−2. The Cu foil with slurry was vacuum-dried at 80°C overnight and cut into several disks with a diameter of 10 mm. Li metal foil served as the counter electrode, and Celgard 2400 was used as the separator. The electrolyte consisted of 1 mol L−1 LiPF6 in a mixture of diethyl carbonate (DEC) and ethyl carbonate (EC) in a 1:1 volume ratio. The galvanostatic charge/discharge measurements were carried out at current densities of 0.5 C, 1 C, 2 C, 5 C, and 10 C (1 C = 754.8mA g−1) within a voltage range of 0.01–3 V. Cyclic voltammetry (CV) tests were performed at a scanning rate of 0.1 mV s−1 between 0.01–3 V using an OPENSENSE machine. Electrochemical impedance spectroscopy (EIS) was performed using the same equipment as for the cyclic voltammetry (CV) tests.
To determine the morphology and structure of materials including nano Si particles, carbon C45, graphene, and synthetic materials using the method of mechanical ball-milling, the X-ray diffraction (XRD) analysis was conducted and the results are shown in Fig. 1a. The commercial nano Si material exhibited five characteristic diffraction peaks at 28.4°, 47.3°, 56.1°, 69.1°, and 76.3° corresponding to the (111), (220), (311), (400), and (331) planes of crystalline Si (JCPDS card No. 00-027-1402), respectively. In addition, carbon C45 in an amorphous state does not exhibit any diffraction peaks, while graphene shows a diffraction peak observed at 26.5° and 54.5 corresponding to the (002) and (004) crystal planes. Moreover, the XRD results of Si/C45:Gr composite material showed diffraction peaks that corresponded to the characteristic diffraction peaks of both commercial Si and graphene. Furthermore, from B1 to B4, an increasing trend in the peak intensity of the (002) and (004) crystal planes of graphene was observed, which correlates with the increasing proportion of graphene in the composite material. From the X-ray diffraction (XRD) results of the ball-milling composite materials, it can be concluded that the materials’ structure remains after synthesis.
Fig. 1b displays the Raman spectrum of the composite materials, highlighting the prominent D and G peaks. These peaks were found to be the most dominant features among all the composite materials analyzed. The D band in Raman spectroscopy is associated with carbon materials’ disorder and structural defects, while the G peak arises from the stretching vibrations of carbon-carbon bonds in the hexagonal lattice structure. The intensity ratio of the D-band to the G-band, denoted as ID/IG, is a widely used parameter to evaluate the degree of structural disorder in carbon materials. A higher ratio indicates a higher density of defects or disorders in the material. The presence of D and G peaks at 1340 and 1582 cm−1, respectively, was observed in all the samples. These two signals are indicative of graphene’s characteristic properties. Furthermore, the ID/IG ratios of all Si/C45:Gr composites exhibit an approximate value of 1.1. The XRD and Raman spectroscopy analyses confirmed the presence of graphene in all the samples, providing further evidence of their inclusion.
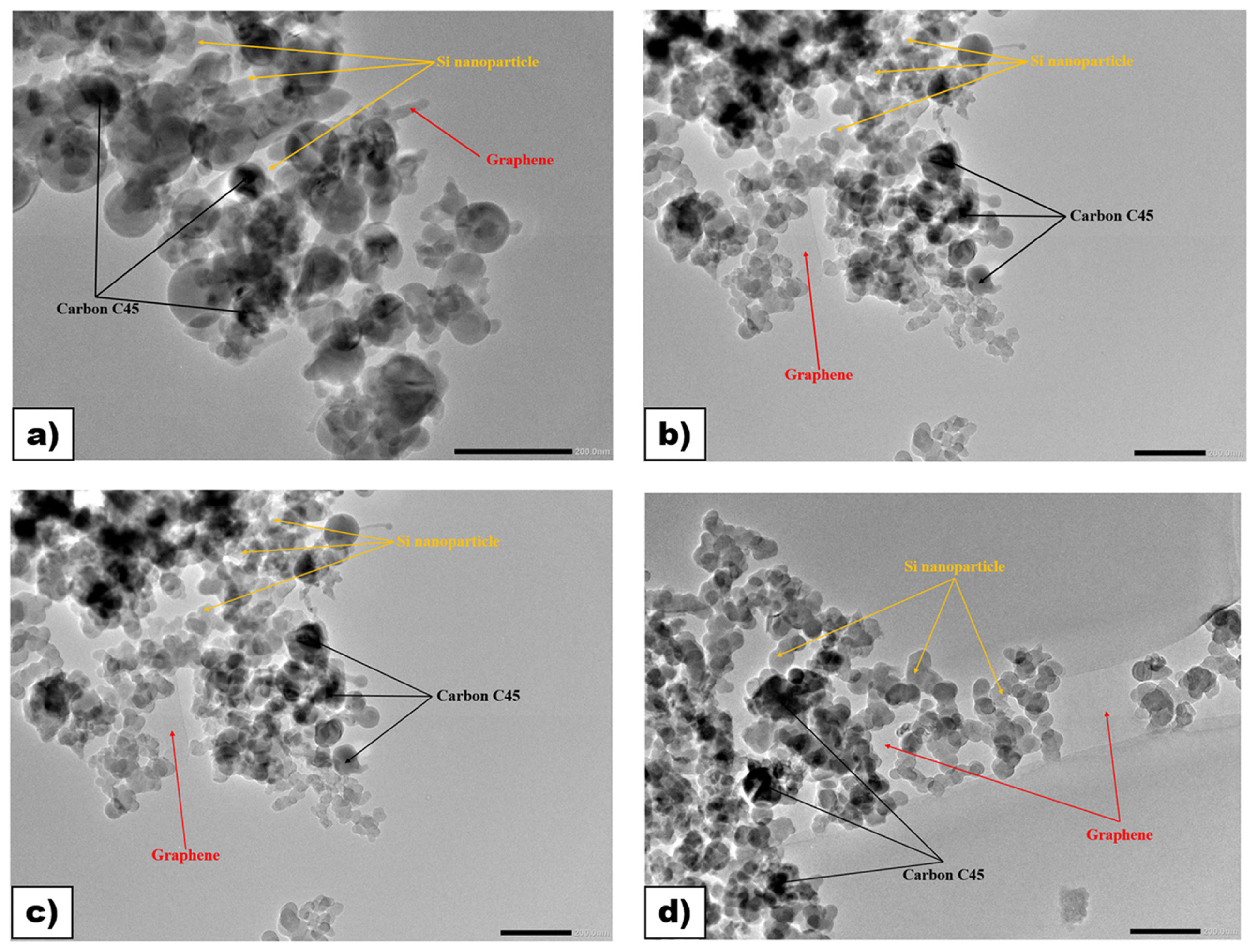
The morphologies of Si/C composites have been investigated using TEM techniques. In Fig. 2, TEM images suggest that the composite is predominantly composed of carbon (approximately 80 wt%), with carbon materials covering 20 wt% of Si nanoparticles (Si powders contain nanosized Si particles that are spherical in shape, with individual particle sizes ranging from 80 to 100 nm). Moreover, this demonstrates that Si powders are surrounded by amorphous carbon, indicating that nanocrystalline Si particles are uniformly embedded in an amorphous carbon matrix during the ball-milling synthesis process. Furthermore, as the number of graphene increases and the amount of carbon C45 decreases (from B1 to B4), more Si nanoparticles can be observed, along with a more defined appearance of graphene sheets.
To gain a better understanding of the surface changes in Si/C composites synthesized by a simple ball-milling method, scanning electron microscopy (SEM) was employed, and the images are shown in Fig. 3a–d. All composite materials exhibited irregular particle shapes and morphologies due to the grinding process. In addition, SEM analysis allows us to predict that the Si nanoparticles have a spherical shape with an approximate size of 100 nm, which is consistent with the results obtained from TEM.
To further investigate the superior electrochemical performance of Si/C composite materials, we observed the morphology of Si/C45:Gr by cross-section SEM before and after 100 cycling (Fig. 3e–h). Initially, the thickness of the coating layer has an average thickness ranging from 30 to 34 μm. After the charging process, all four materials (B1–B4) show changes in the thickness of the coating layer due to the volume expansion of Si particles. The cross-section SEM results reveal that material B1 has the least thickness variation (~40 μm), followed by materials B2 and B3 (~45–51 μm), and finally, material B4 with the largest variation (~55 μm). Overall, all four materials exhibit relatively small changes in the thickness of the coating layer, ranging from 30 to 34 μm to 40 to 55 μm, corresponding to a volume change of about 100% compared to the initial state. This is consistent with the disadvantage of Si, which undergoes large volume changes during electrochemical cycling.
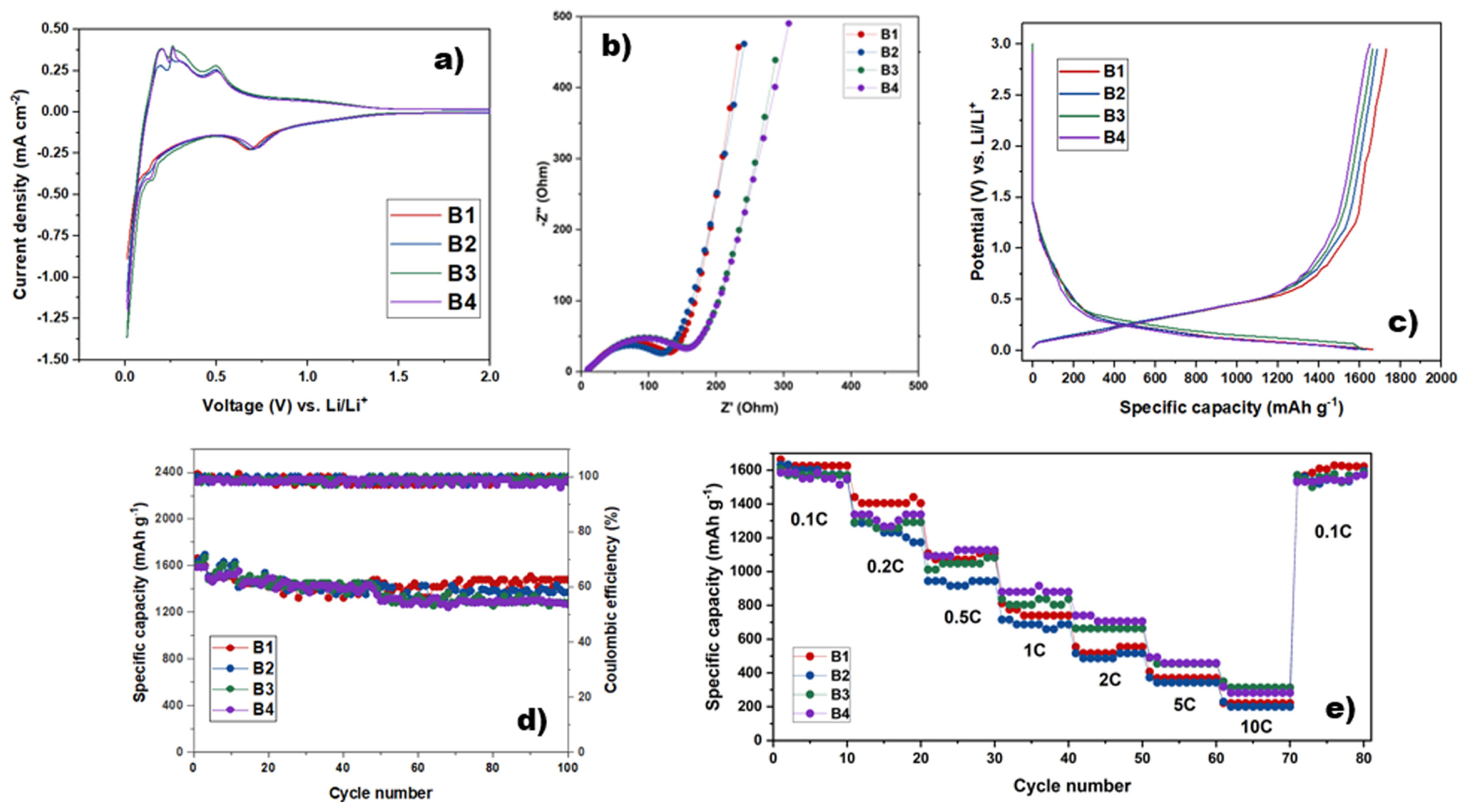
To investigate the electrochemical performance of Si/C45:Gr materials, cyclic voltammetry (CV) was conducted with a scan rate of 0.01 mV s−1 (Fig. 4a). The charge curves of Si/C composites exhibited voltage plateaus between 0.35 and 0.45 V. The formation of the solid electrode interphase (SEI) layer caused a change in the inflection section. Fig. 4a shows that the appearance of the SEI film resulted in a sloped and broad peak located at around 0.68 V vs. Li/Li+ and led to the irreversible loss of lithium. On the cathodic branch, there were two other peaks at around 0.01 and 0.15 V, caused by Li alloying to LixSi phase. Moreover, the values of two peaks at approximately 0.25 and 0.5 V could be attributed to the decomposition of LixSi phase at the anode peak.
Fig. 4b presents the results of electrochemical impedance (EIS) testing on four types of Si/C45:Gr samples. At high frequencies, the semicircle observed can be attributed to the resistance related to charge transfer and SEI formation at the interface of the composites. At low frequencies, a straight line is indicative of Li+ diffusion in the anode material. The semicircle of Si/C materials increased with increasing graphene content due to the formation of a stable SEI layer during the charge/discharge process and the dense contact between carbon (both super C45 and graphene) with Si particles. Additionally, the Li+ transfer impedance decreased significantly from B4 to B1 due to the good conductivity of carbon super C45. In summary, the EIS results indicated that the addition of carbon, especially carbon C45, improved the electrochemical performance of Si/C anodes by enhancing the Li+ transfer and reducing the resistance related to charging transfer and SEI formation at the interface of the composite. This was consistent with the improved cycling stability and rate performance observed in the charge/discharge tests.
The electrochemical cycling test of Si/C45:Gr composites was compared to that of Si/C anodes, and the results were presented in Fig. 4c–e. The initial charge and discharge profiles of Si/C anodes were shown in Fig. 4c, the first specific discharge capacities of B1 to B4 were slightly decreased, with values of 1663.2, 1632.4, 1606.9, and 1586.2 mAh g−1, respectively. After the increase of graphene proportion in the composite, the initial Coulumbic efficiency (CE) of B1 to B4 decreased from 101.3% to 98.7%. The high CE is supposed to enhance the conductivity by reconnecting fragmentation C45 networks. After 100 cycles at the current density of 0.1 C, all the samples underwent a slow capacity fading, with retention of approximately 80% (Fig. 2e). Furthermore, the CE after 100 cycles showed a similar trend to these initials, with a CE of approximately 99.0%. The first CE and after 100 cycles exhibited an identical trend because the carbon C45 is not enough to cover as a protective coating for Si particles.
B1 showed the best cycling performance, with a specific capacity of 1480 mAh g−1, which was higher than that of B2 (1370 mAh g−1), B3 (1284 mAh g−1), and B4 (1268 mAh g−1). The rate performance of Si/C45:Gr composites was also tested at various current densities, as shown in Fig. 4e. The specific capacities of all samples decreased with increasing current density. Even at a high current density of 10 C, the specific capacities of B1, B2, B3, and B4 were approximately 300 mAh g−1, respectively. Upon returning to 0.1 C after high-speed discharge/charge, the capacity of the Si/C composite samples recovered to approximately the original capacity. This observation confirms the ability of Si/C composite material to withstand high-speed discharge/charge conditions, indicating its potential for high-rate applications in lithium-ion batteries. The swift restoration of the initial specific capacity when the current density drops to 0.1 C provides additional evidence of the beneficial effect of the carbon in the Si/C composite. These results demonstrated that the Si/C45:Gr composites exhibited good electrochemical performance, with a higher specific capacity, better cycling stability, and better rate performance, due to the presence of abundant channels for lithium-ion transport in the carbon.
The results obtained from our study demonstrate the good electrochemical performance of Si/C45:Gr materials during cycling. This can be attributed to the protective coating of the carbon C45 layer, which effectively reduces volume changes, prevents nanoparticle aggregation, and buffers expansion stress. Additionally, the stability of the solid electrolyte interface (SEI) layer is crucial for maintaining stable cycles, resulting in excellent electrochemical performance during lithiation and de-lithiation. Moreover, the graphene component in the composite provides a conductive network that enhances conductivity and promotes Li+ transportation during charge/discharge processes. In conclusion, the synthesis of Si/C45:Graphene anode materials through ball-milling Si nanoparticles with carbon C45 (70 wt%) and graphene (10 wt%) significantly enhances their electrochemical performance, making them highly promising for commercial applications in Si/C anodes.
In conclusion, Si/C composites with the incorporation of carbon C45 and graphene have been successfully synthesized using a simple ball-milling method. The C45/Gr ratio has been found to significantly impact the electrochemical performance of the composites. The graphene sheets in the composites help to improve the conductivity by reconnecting broken C45 networks, and carbon C45 also acts as a protective coating for Si particles, mitigating their volume expansion. As a result, the Si/C45:Gr composites exhibit enhanced electrochemical performance, including higher capacity and improved cycling stability. This study highlights the promising potential of Si/C45:Gr composites as anode materials for LIBs and provides insights into the effective role of graphene in improving the performance of Si-based anode materials. The straightforward and scalable fabrication process of silicon anode materials makes them highly promising for practical applications in commercial lithium-ion batteries.
Acknowledgments
This work is funded by the Ministry of Science and Technology (MOST) under grant NĐT/CN/21/23.
Fig. 1
(a) X-ray diffraction pattern of C45, Graphene, Si/C45:Gr composites (B1–B4) and (b) Raman spectra of B1 to B4 samples.
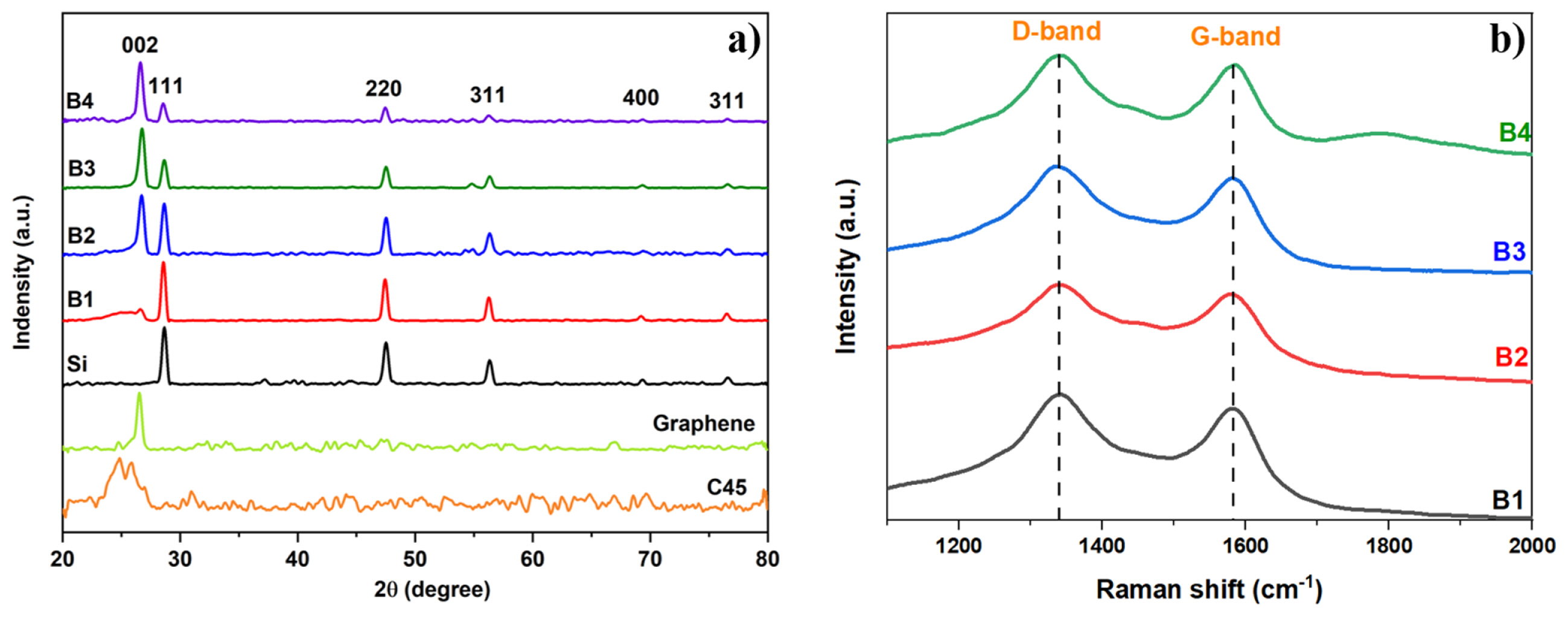
Fig. 3
(a–d) SEM image of synthesized powders and (e–h) cross-section SEM image of the Si/C45:Gr composites before and after 100 cycles.

Fig. 4
(a) Cyclic voltammetry curves at a scan rate of 0.1 mV s−1 for the cut-off voltage of 0.01–3.0 V vs. Li/Li+; (b) Electrochemical impedance spectra and equivalent circuit of the composites Si/C45:Gr; (c) The first charge/discharge curves at a galvanostatic current density of 0.1 C while cycling between 0.01–3.0 V vs. Li/Li+; (d) Cycling performance of the composites anode materials at 0.1 C; and (e) Rate performance at various currents densities of 0.1 C, 0.2 C, 0.5 C, 1.0 C, and 2.0 C for the Si/Gr/C45 electrodes.
References
[1] A. Brecher, Transit bus applications of lithium-ion batteries: Progress and prospects. In: G Pistoia editors. Lithium-Ion Batteries. Elsevier B.V, 2014. p.177–203.
[2] G. Zubi, R. Dufo-López, M. Carvalho and G. Pasaoglu, Renew. Sustain. Energy Rev, 2018, 89, 292–308.

[3] H. A. Nguyen, P. P. N. Le, L. T. N. Huynh, T. V. Man and M. L. P. Le, VNUHCM Journal of Natural Sciences, 2019, 3(1), 46–54.
[5] C. K. Chan, H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nature Nanotech, 2008, 3(1), 31–35.


[7] G. Zheng, S. W. Lee, Z. Liang, H.-W. Lee, K. Yan, H. Yao, H. Wang, W. Li, S. Chu and Y. Cui, Nature Nanotech, 2014, 9(8), 618–623.


[9] J. Wang, Y. Cui and D. Wang, Adv. Mater, 2019, 31(38), 1801993.
[10] P. U. Nzereogu, A. D. Omah, F. I. Ezema, E. I. Iwuoha and A. C. Nwanya, Appl. Surf. Sci. Adv, 2022, 9, 100233.

[11] K. Xu, A silicon future. In: C Ban, K XuIn: Lithium–ion Batteries Enabled by Silicon Anodes IET Digital Library. 2021, 405.

[12] X. Ding, H. Wang, X. Liu, Z. Gao, Y. Huang, D. Lv, P. He and Y. Huang, RSC Adv, 2017, 7(26), 15694–15701.

[13] S. Suresh, Z. P. Wu, S. F. Bartolucci, S. Basu, R. Mukherjee, T. Gupta, P. Hundekar, Y. Shi, T.-M. Lu and N. Koratkar, ACS Nano, 2017, 11(5), 5051–5061.

[14] X. Ding, X. Liu, Y. Huang, X. Zhang, Q. Zhao, X. Xiang, G. Li, P. He, Z. Wen, J. Li and Y. Huang, Nano Energy, 2016, 27, 647–657.

[17] M. J. Loveridge, M. J. Lain, Q. Huang, C. Wan, A. J. Roberts, G. S. Pappas and R. Bhagat, Phys. Chem. Chem. Phys, 2016, 18, 30677–30685.

[18] Q. Huang, M. J. Loveridge, R. Genieser, M. J. Lain and R. Bhagat, Sci. Rep, 2018, 8(1), 1386.
[19] S.-L. Chou, J.-Z. Wang, M. Choucair, H.-K. Liu, J. A. Stride and S.-X. Dou, Electrochem. Commun, 2010, 12(2), 303–306.

[20] I. H. Son, J. H. Park, S. Park, K. Park, S. Han, J. Shin, S.-G. Doo, Y. Hwang, H. Chang and J. W. Choi, Nat. Commun, 2017, 8(1), 1561.
[21] J. Yang, B. F. Wang, K. Wang, Y. Liu, J. Y. Xie and Z. S. Wen, Electrochem. Solid–State Lett, 2003, 6(8), A154.

[22] Y. Liu, Y. Zhang, Y. Du, Y. Zhou, G. Hou, T. D. Lam, Y. Yang, F. Yuan and X. Wang, Energy Technol, 2019, 7(7), 1900037.
[23] S. Palumbo, L. Silvestri, A. Ansaldo, R. Brescia, F. Bonaccorso and V. Pellegrini, ACS Appl. Energy Mater, 2019, 2(3), 1793–1802.

[24] X. Zhang, D. Wang, X. Qiu, Y. Ma, D. Kong, K. Müllen, X. Li and L. Zhi, Nat. Commun, 2020, 11(1), 3826.
- TOOLS