1. Introduction
The increasing application of lithium-ion cells in transportation sectors, including electric vehicles and transport vessels [1ŌĆō6], has spurred a need for the facile refueling of battery systems to ensure enhanced usability [7ŌĆō10]. A major challenge to achieving quick-charging capabilities in lithium-ion battery systems stems from the negative-electrode side. Li plating on the negative-electrode surface during high C-rate applications significantly compromises cell performance [11]. The Li metal, plating in a dendritic form on the negative-electrode surface, can penetrate the polymeric separator, leading to an internal cell short, subsequently causing the battery packsŌĆÖ thermal runaway. Consequently, it is crucial to inhibit Li plating on the negative-electrode surface to enhance lithium-ion battery performance during quick charging. Various strategies including electrolyte engineering [1,3,12], charging procedure modification [13,14], electrode architecture design [8,15], and the replacement of graphite toward silicon negative [1,14] electrodes have been implemented to decrease charging time. Silicon, with a specific capacity 10 times higher than graphite, facilitates a thinner electrode structure for the same areal capacity, making the silicon negative electrode-based system a promising approach for improving both the quick charge performance and energy density of lithium-ion cells. However, significant volumetric expansion that the silicon electrode undergoes during cycling escalates cell impedance. The failure of the silicon active materials greatly deteriorates the electrode structure, and hence the mitigation of the electrode failure is conducted by binder [16], electrolyte additives [1,17ŌĆō20] and the state-of-charge control [21ŌĆō23]. As a result, the pre-used state-of-charge significantly impacts the subsequent quick-charging performance [14]. This study introduces the use of fluoroethylene carbonate (FEC) as an electrode fatigue suppressor to mitigate fatigue during low C-rate cycling of the silicon electrode. The expected outcome is a considerable slowdown in the deterioration of silicon-based electrodes, owing to the effective formation of a viscoelastic solid electrolyte interphase (SEI) film by FEC [24]. The subsequent decrease in cell impedance after cycling, as a result of mitigated mechanical electrode degradation, is anticipated to improve quick-charge performance after cell aging. The paper will evaluate the performance of a Si-based negative electrode combined with a Ni-rich positive electrode (NCM811) in a pouch cell, with and without the use of FEC. A comparative analysis is undertaken to confirm that the SEI film properties significantly alleviate electrode fatigue, a major hindrance to quick-charge performance.
2. Experimental
2.1 Preparation of electrode and laminated pouch cell
Lithium nickel manganese cobalt oxide (LiNi0.8 Co0.1Mn0.1O2, NCM811, EcoPro BM) was used to fabricate the positive electrode, with carbon black (Super P, Timcal) and poly(vinylidenefluoride), as the binder, in a 95:2.5:2.5 wt.% ratio. The mixed powder was blended and then dispersed in N-methyl-2-pyrrolidone (Sigma-Aldrich). The slurry was then applied to a 15 ╬╝m aluminum foil. The coated electrode was initially pre-dried at 80┬░C for 5 h before undergoing a 12 h heat treatment at 120┬░C in a vacuum. The negative electrode was produced using SiO (DMSO, Daejoo Electronic Materials), flake graphite (SFG6, Timcal), and a poly(acrylic acid)/poly(vinyl alcohol) (PAA, Sigma-Aldrich, Mw = 250,000/PVA, Sigma-Aldrich, Mw = 19,000ŌĆō23,000, 9:1 ratio) binder in DI water with a 7:2:1 wt.% ratio. This mixture was applied to a 20 ╬╝m thick copper foil and vacuum dried at 80┬░C for 12 h. Subsequently, it was heated at 150┬░C for 1 h in a vacuum oven to cross-link the PVA and PAA binders. The positive electrode, PE (NH616, Asahi Kasei) separator, and negative electrode were assembled to form a pouch-type cell. Given a charge (de-lithiation) areal capacity for the negative electrode of 2.08 mA h cmŌłÆ2, the cell had a specified negative-to-positive electrode capacity ratio (NP ratio) of 1.04. After thermal sealing, the cells were filled with 1.0 M LiPF6 in EC/EMC (3:7 v/ v) and 1 wt.% of FEC-added electrolyte (Dongwha electrolyte), and then aged at room temperature for 24 h. To stabilize the surface film formed on the electrode, a 0.1 C constant current (CC) charge was applied for 3 h, followed by one day of rest at ambient and high temperatures. Galvanostatic charge-discharge pre-cycling (TOYO, Japan) was conducted using a 0.33 C CC-CV charge (0.05 C current cut-off) and a 0.33 C discharge after stabilization. Following pre-cycling, a 0.5 C CC-CV charge (0.05 C current cut-off) and a 0.5 C discharge within a state-of-charge (SOC) range of 0ŌĆō100 (4.2ŌĆō2.5 V) were used for normal C-rate cycling.
2.2 Ex-situ analysis of cycled electrodes
X-ray photoelectron spectroscopy (XPS, Scientific K-ALPHA, Thermo Fisher) and scanning electron microscopy (SEM, JEOL JSM-IT200) were utilized in a dry room to examine the chemical composition and structure of the negative electrode after quick and normal charge cycles. The cycled electrodes were disassembled in an Ar-filled glove box, cleansed with ethyl methyl carbonate (EMC, Dongwha Electrolyte), and vacuum dried for 12 h.
2.3 Electrochemical impedance spectroscopy
To measure the resistance of the SiO electrode following quick-charge cycling, a negative-electrode symmetric cell was assembled using fresh 1.0 M LiPF6 in EC/EMC (3:7 v/v). A constant current-constant voltage (CC-CV) discharge step (down to 2.5 V) was employed to fully delithiate the cycled SiO electrode and stabilize the open-circuit voltages (OCVs). Potentiodynamic electrochemical impedance spectroscopy (PEIS) experiments were conducted with a 5.0 mV voltage perturbation over a frequency range of 10.0 mHz to 3.0 MHz. The experiment was carried out using a Li/Li/SiO three-electrode configuration in a beaker cell.
2.4 Electrochemical quartz crystal microbalance
Electrochemical quartz crystal microbalance (EQCM, Seiko EG&G) tests were performed using a custom-made QCM well cell. A Pt-sputtered quartz crystal served as the working electrode, while 200 ╬╝m Li metal (Honjo metal) constituted the counter and reference electrodes. The voltage range for the test was set from open-circuit voltage to 0.3 V (vs. Li/ Li+).
3. Results and Discussion
Fig. 1 displays the cycleability and charging voltage profiles of the SiO/NCM811 cell in both base and FEC-enhanced electrolytes. In 0.5 C CC-CV conditions, a charging time of 120 min was recorded, and the constructed SiO/NCM811 cells exhibited the capacity to function at their full designed capacity (Fig. 1a). The cycleability, derived from both base and FEC-enhanced electrolytes at 0.5 C cycle, demonstrates the enhancement in capacity retention due to robust SEI formation on SiO electrodes, with identical initial cell capacity (Fig. 1b). Alterations in the properties of the SEI film induced by FEC led to a 20% increase in capacity retention. The FEC additive is predicted to prevent deterioration, subsequent Li-ion consumption, and trapping at the negative electrode, given the augmented capacity retention of the FEC-enhanced electrolyte boosts cell cycleability. Fig. 1c presents the 6-min chargeable procedure along with the corresponding voltage profiles. The pouch cell underwent an intermittent current step designed to prevent Li deposition during charging, with a cut-off voltage of 4.2 V further refining the end condition. This application of the quick-charge procedure significantly curbs Li plating during the cycle. Fig. 1d illustrates quick-charge cycleability. The FEC-enhanced electrolyte outperformed the base electrolyte in terms of quick-charge performance as the FEC additive safeguarded against deterioration of the negative electrode. Notably, even with high utilization of the SiO electrode, the FEC-enhanced cell displayed superior capacity retention compared to the base electrolyte.
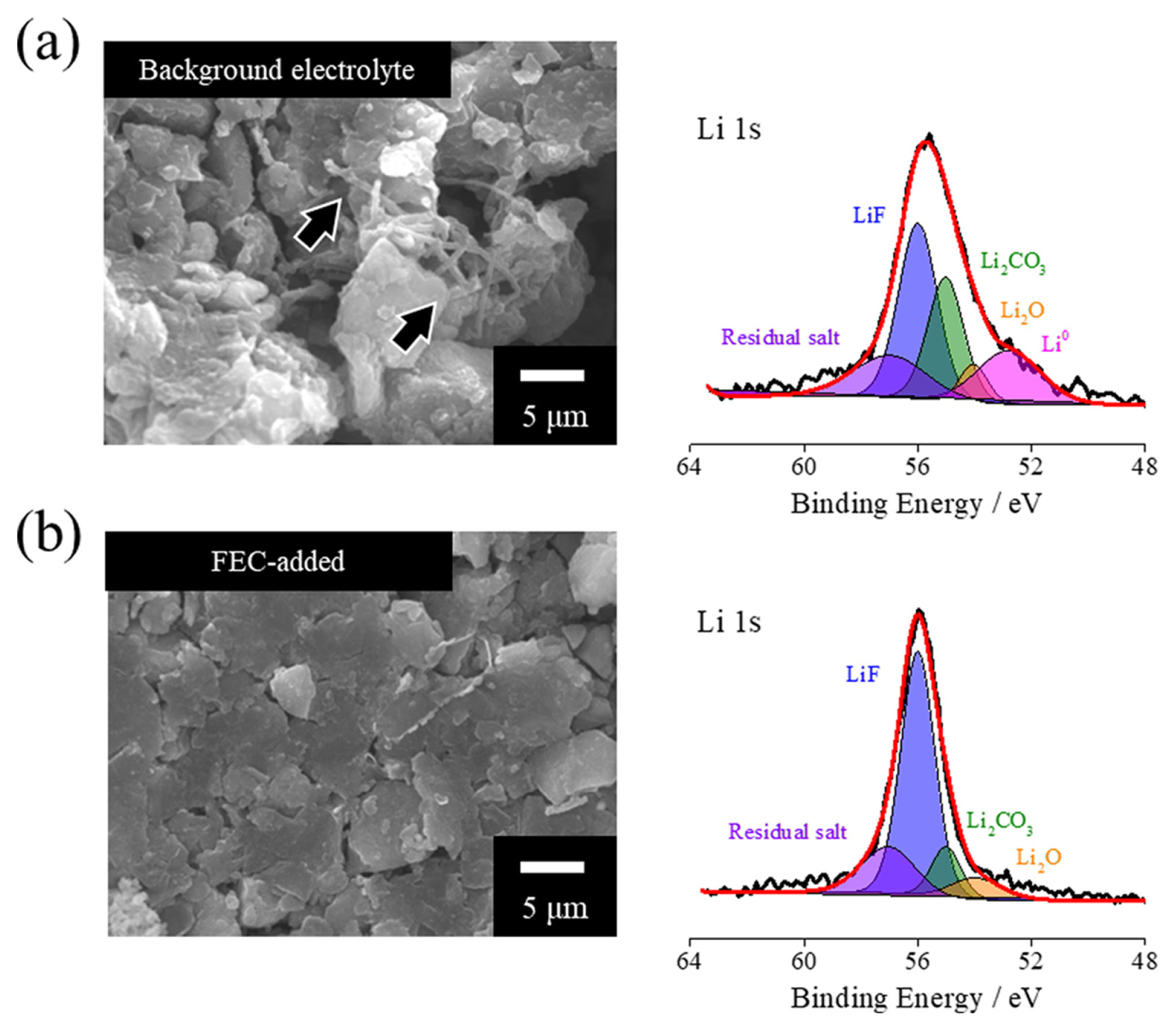
Following the quick-charge cycles, ex-situ morphological and chemical composition analyses of the SiO electrodes were performed (Fig. 2). The topmost SEM image from the cell with base electrolyte reveals rod-like deposits on the electrode surface, and the Li 1s XPS spectrum indicates a high abundance of metallic Li on the electrode surface [25] (Fig. 2a). Hence, the failure of cycleability in high C-rate applications can be attributed to Li deposition from the base electrolyte on the negative-electrode surface during quick charging. In contrast, the SiO electrode from the cell treated with the FEC-enhanced electrolyte displayed a clean electrode surface, and the XPS spectrum revealed no significant signals of plated Li. The inclusion of FEC led to the formation of an LiF-based SEI film, aligning with previous reports [26ŌĆō29]. Consequently, the prevention ability of FEC from the Li deposition on the negative-electrode surface considerably enhanced the quick-charge cycleability. The C 1s and F 1s spectra (Fig. S1) depicts corresponding peaks demonstrated at Li 1s spectra, and the exposure of Si-C peak from the destruction of deposited SEI is severely observed with background electrolyte, which indicates the reinforcement of SEI film by FEC additive.
Following the 100th 0.5 C cycle, electrochemical impedance spectroscopy analysis utilizing a three-electrode setup was performed to identify the source of differences in Li deposition (Fig. 3). The Li/Li/SiO cell is assembled at the fully-delithiated state of SiO electrode, and hence the semi-circle from the Nyquist plot is from the SEI resistance (RSEI). The RSEI,background was 137 ╬® mg, whereas the RSEI,FEC was 102 ╬® mg; which indicates the interphase degradation is suppressed with FEC incorporation. As a result, following the 100th 0.5 C cycle with the FEC additive, the kinetics of the Li-ion alloying reaction with the SiO electrode were well preserved. The maintained lithiation kinetics of the SiO electrode after the 100th cycle with FEC additive suppresses Li-ion plating on the SiO surface, as Li-ion plating on the negative electrode occurs under conditions of insufficient lithiation kinetics, represented by the RSEI resistance.
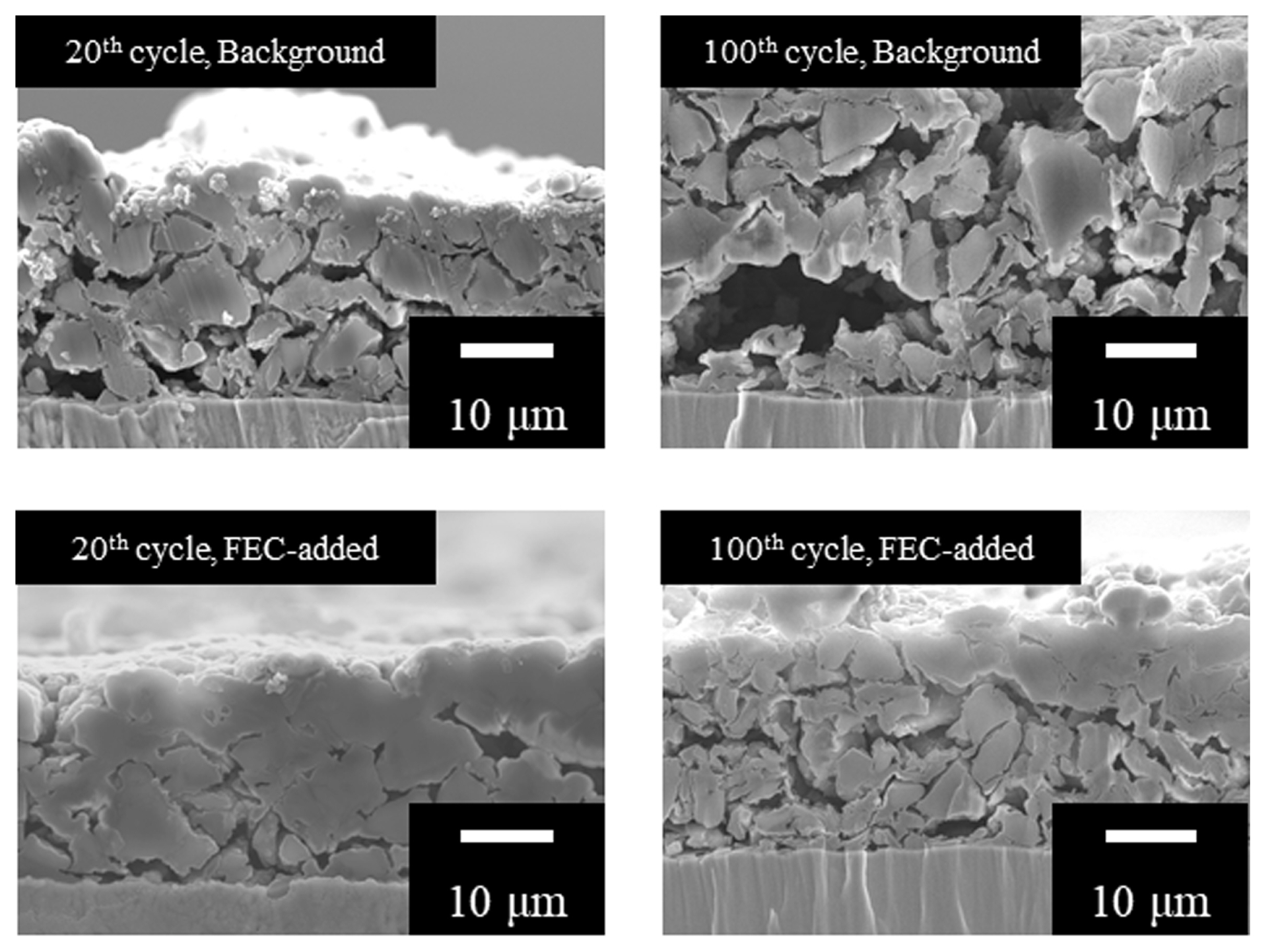
Cross-sectional SEM images of the SiO electrode, treated with base and FEC-enhanced electrolytes after the 20th and 100th cycles, were used to investigate electrode fatigue (Fig. 4). After 20 cycles, the SiO electrode treated with the base electrolyte exhibits significant electrode deterioration and electrically disconnected particles. A notable void within the electrode structure impedes efficient electronic transfer from the current collector. After the 100th cycle, the structural failure of the electrode treated with the base electrolyte continues to worsen. Detached particles and a void, 10 ╬╝m in size, develop within the electrode. In contrast, the use of FEC significantly mitigates electrode expansion and void formation. The 20th cycle SEM images of the electrode treated with FEC reveal well-connected SiO particles to the current collector and reduced void formation inside the electrode. FEC addition manages to control the morphological failure of the electrode, thereby limiting the increase in electrode thickness. Improved charge transfer at the SiO surface, resulting from electrically connected particles, reduces the number of Li ions deposited on the surface of the negative electrode. Moreover, FEC addition impedes the increase in electrode thickness, facilitating easier diffusion of Li ions into the negative electrode compared to the substantially expanded electrode treated with the base electrolyte. Therefore, the primary mechanism behind the enhancement of quick-charge performance via FEC addition is the prevention of electrode fatigue caused by volumetric expansion and subsequent electrolyte breakdown on the newly exposed SiO surface.
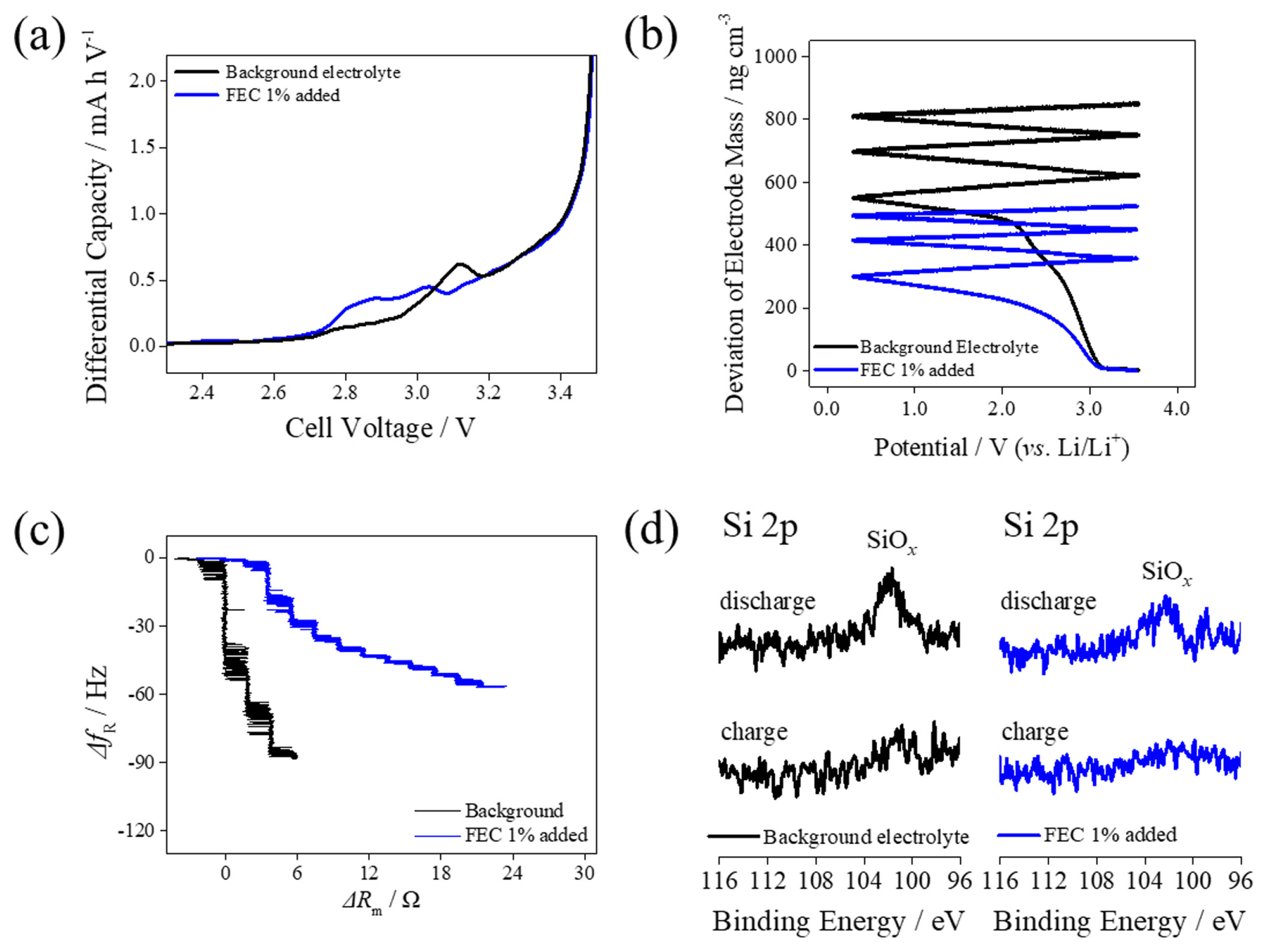
Given that the introduction of FEC counters fatigue induced by mechanical degradation of the electrode, we proceeded to analyze the mechanical characteristics of the SEI film deposited on the SiO electrode (Fig. 5). The differential capacity plots obtained during the formation of the SiO/NCM811 cell revealed distinct peaks preceding ethylene carbonate reduction, attributed to the reduction of the FEC additive (Fig. 5a). Consequently, it is anticipated that the composition of SEI films is influenced by FEC. The passivation ability of the FEC-derived surface film was confirmed using EQCM measurements (Fig. 5b). Early formation of the SEI film by FEC suppressed further electrolyte decomposition during initial exposure to low-voltage regions. This led to a noticeable decrease in the final electrode mass following the initial negative voltage sweep. Specifically, a 50% reduction in final mass was observed with the addition of FEC compared to the base electrolyte. Furthermore, an increase in mass was observed during continuous exposure to low-voltage regions, indicative of additional electrolyte decomposition after SEI formation. This was attributable to the enhanced passivation ability of the FEC-derived film. The mechanical properties of the SEI film on the SiO electrode were evaluated by comparing the motional resistance (ΔRm) against the resonant frequency (ΔfR) slope. A previous study established that a sharp increase in motional resistance with a rising resonant frequency is due to stiff film deposition [30]; therefore, the SEI film developed from the FEC additive is more viscoelastic than those formed from the base electrolytes. The resilience of the SEI film on the SiO electrode during the expansion and shrinkage of the active material was further investigated by ex-situ XPS measurements (Fig. 5d). The SEI film is deposited on the SiO electrode when the SiO/NCM811 pouch cell is charged to 4.2 V, rendering the signal from the Si 2p spectra unidentifiable [1]. Conversely, a distinct SiOx peak from Si 2p spectra is observed at the SiO electrode after discharge to 2.5 V, as the mechanical degradation of the SEI film on the SiO electrode becomes prominent with the base electrolyte. The SEI film derived from the electrolyte with FEC minimized the exposure of the SiO surface, whereas the SEI film obtained from the base electrolyte experienced mechanical degradation. Consequently, enhancing the properties of the SEI film with FEC reduced the exposure of the bare SiO electrode surface during a normal C-rate cycle, decreasing the additional formation of the SEI film and resultant electrode fatigue. Therefore, the incorporation of the FEC additive effectively reduces electrode fatigue, improves quick-charge performance, and decreases Li deposition on the negative-electrode surface during high C-rate operations.
4. Conclusions
In this study, we conducted a comparative analysis of the effects of SEI reinforcement on the quick-charge performance of the SiO electrode. The addition of FEC to the SiO electrode led to notable electrode fatigue, which can be attributed to the viscoelastic characteristics of the FEC-derived SEI film. The FEC-derived SEI film, due to its high tolerance to volumetric strain, effectively suppresses the exposure of the bare SiO surface during normal C-rate cycling. As a result, the mechanical failure of the electrode induced by additional electrolyte decomposition is substantially reduced with the incorporation of FEC. The FEC additive ensures well-preserved electrical connectivity between the active materials and the current collector after cycling. Consequently, Li deposition during quick-charge cycles after aging at normal C-rate cycling is significantly improved with FEC. In conclusion, reinforcing the interfacial characteristics of the SiO electrode is crucial for maintaining the kinetics of overall battery system. This is because the degradation of the SEI film can adversely affect the electrode architecture.