|
 |
- Search
| J. Electrochem. Sci. Technol > Volume 14(3); 2023 > Article |
|
Abstract
The global energy storage markets have gravitated to high-energy-density and low cost of lithium-ion batteries (LIBs) as the predominant system for energy storage such as electric vehicles (EVs). High-Ni layered oxides are considered promising next-generation cathode materials for LIBs owing to their significant advantages in terms of high energy density. However, the practical application of high-Ni cathodes remains challenging, because of their structural and surface instability. Although extensive studies have been conducted to mitigate these inherent instabilities, a two-step process involving the synthesis of the cathode and a dry/wet coating is essential. This study evaluates a one-step β-Li2SnO3 layer coating on the surface of LiNi0.8Co0.2O2 (NC82) via the thermal segregation of Sn owing to the solubility limit with respect to the synthesis temperature. The doping, segregation, and phase transition of Sn were systematically revealed by structural analyses. Moreover, surface-engineered 5 mol% Sn-coated LiNi0.8Co0.2O2 (NC82_Sn5%) exhibited superior capacity retention compared to bare NC82 owing to the stable surface coating layer. Thus, the developed one-step coating method is suitable for improving the properties of high-Ni layered oxide cathode materials for application in LIBs.
Although the global warming trend has been ongoing for a long time, its rate has increased significantly over the past 100 years owing to anthropogenic greenhouse gas emissions from burning fossil fuels [1–5]. Consequently, many countries have taken responsibility and worked together to address the common global issues of climate change. Revolutionary changes are underway in the transport sector, along with global efforts toward more sustainable energy sources, which will significantly lower the global carbon footprint. Moreover, the development of EVs by global automakers has the potential to dramatically reduce greenhouse gas emissions [6–10]. EVs have pursued technological advances that internal combustion engine vehicles have pioneered for decades. Among the various components of EVs, lithium-ion batteries (LIBs) are crucial for their successful realization [11–14]. The high energy density and lightweight of LIBs are the most important characteristics for promoting their application in EVs [15–17]. Recently, EV manufacturers have been exploring methods to further improve the energy density of LIBs, and thereby extend their driving range. To realize this, high-Ni cathode materials, such as LiNi1−x−yCoxMnyO2 (NCM), LiNi1−x−yCoxAlyO2 (NCA), and LiNi1−x−y−zCoxMnyAlzO2 (NCMA), are considered key materials [18–24].
However, there are challenges associated with high-Ni cathodes, including structural and surface instabilities. The main cause of the structural instability of high-Ni cathodes, such as microcrack formation, is that the lattice parameter along the c-axis of the cathode material contracts during charging, resulting in stress and strain within the grain structure, and consequently, an increase in surface reactivity [25–28]. Moreover, electrolyte decomposition at the surface of high-Ni cathodes originates from the Ni4+ ions generated at high voltages [29–31]. Extensive studies, such as surface coating, have been conducted to address the critical issues of high-Ni cathodes [32–36]. Generally, a two-step process is required to coat high-Ni cathodes. However, the practical application of the two-step process for coating high-Ni cathodes has several challenges, including long processing time, low efficiency, and high cost. Recently, a one-step plane-selective coating method for LiCoO2 electrode materials was used to prepare a conformal coating layer only on the (001) plane of LiCoO2 by the thermal segregation of Li2SnO3, which is induced by changes in the solubility of Sn in the electrode material as a function of temperature [37]. However, one-pot coatings of high-Ni cathodes by thermal segregation have rarely been investigated thus far.
This study presents the feasibility of a one-pot coating method for LiNi0.8Co0.2O2 (NC82) high-Ni layered oxide cathodes by surface segregation based on the limitations of dopant solubility in cathodes as a function of synthesis temperature. The dependence of Sn dopant solubility on the synthesis temperature in NC82 is systematically evaluated based on structural changes determined by X-ray diffraction (XRD). The Sn surface coating of Li2SnO3 on NC82 is meticulously characterized using scanning transmission electron microscopy (STEM) in the high-angle annular dark field (HAADF) mode and energy dispersive X-ray spectroscopy (EDS). Additionally, the electrochemical performance of the synthesized material is characterized using galvanostatic cycling and rate tests. Moreover, the effect of the Sn coating on NC82 is evaluated by analyzing the surface resistance and structural changes of the cells after cycling using a combination of electrochemical impedance spectroscopy (EIS) and ex situ XRD. Finally, this study hypothesizes that the developed one-pot coating method for high-Ni layered oxide cathodes by thermal segregation of dopants may provide advancements toward mitigating the inherent challenges associated with these materials.
Ni0.8Co0.2(OH)2 precursors were synthesized using a facile sol-gel method with LiNO3, Ni(NO3)2·6H2O, and Co(NO3)2·6H2O in ethanol at a Li/transition metal (TM) molar ratio of 1.03. To synthesize Li2SnO3-coated LiNi0.8Co0.2O2, SnCl2 was added to the as-prepared solution at a TM/Sn molar ratio of 95:5. Subsequently, 0.1 mL of hydrochloric acid (HCl) and citric acid equivalent to the total amount of all cations were added. The solutions were thoroughly stirred for 6 h to obtain transparent gels, which were then dried overnight in a vacuum oven. The as-synthesized powder was heated in air at 300°C for 5 h, and then reheated under an O2 atmosphere at 600–800°C for 10 h at a heating and cooling rate of 2°C min−1.
Structural analyses of all samples were performed by XRD from 10° to 120° using a Bruker D2 PHASER instrument with Cu Kα radiation. Rietveld refinement analyses were performed on the acquired XRD patterns using the TOPAS program. The morphologies of the samples were obtained using scanning electron microscopy (SEM; Carl Zeiss). Transmission electron microscopy (TEM) analyses, including STEM and EDS, were performed using a JEOL JEM-ARM200F instrument.
The cathode was prepared by mixing the electrode material, carbon black (Super P), and poly(vinylidene fluoride) (PVdF) at a mass ratio of 8:1:1 in N-methyl-2-pyrrolidone (NMP) solvent to obtain a slurry. Subsequently, the slurry was cast onto an Al current collector. The electrodes were then dried at 120°C overnight in a vacuum oven. For the coin half cells, the mass loading of the cathode was ~5 mg cm−2. The 1.3 M LiPF6 electrolyte was prepared in an ethylene carbonate (EC)/ethyl methyl carbonate (EMC)/ dimethyl carbonate (DMC) mixture at a volume ratio of 3:4:3 with 2 wt% fluoroethylene carbonate (FEC) as an additive. For the half-cell formation cycle, all samples were cycled between 3 and 4.4 V (vs. Li/Li+) at a rate of C/10 for one cycle, followed by 100 cycles at a rate of C/2 between 3 and 4.4 V (vs. Li/ Li+) at 30°C.
Fig. 1 illustrates the schematic diagram for a one-pot coating method for LiNi0.8Co0.2O2 (NC82) high-Ni layered oxide cathodes by surface segregation based on the limitations of Sn solubility in cathodes as a function of synthesis temperature by a facile solgel and solid-solution method. To explore the solubility of Sn in high-Ni layered oxides, NC82 and 5 mol% Sn-loaded NC82 (NC82_Sn5%) powders were synthesized at different temperatures by a solid-state reaction using precursors prepared via the solgel method. Fig. 2a shows the powder XRD patterns for NC82 and NC82_Sn5% from 600 to 800°C. The XRD pattern of NC82_Sn5% at 600°C corresponded well with that of hexagonal α-NaFeO2 (space group: R-3m), indicating that Sn is homogeneously doped in the host structure without any impurities. Notably, above 650°C, the (002) peak of β-Li2SnO3 (space group: C12/c1) was clearly observed and all the XRD peak intensities increased. Additionally, the solubility limit of Sn in NC82 as a function of temperature was further confirmed by the (003) peak shift. The peak shift in the XRD patterns with Sn doping in the cathode originates from the effect of the difference in the ionic radii of the cations at the TM site. The (003) peak of NC82_Sn5% at 600°C shifted to a lower angle than those of the other NC82_Sn5% samples. The increase in the lattice parameters causes the partial substitution of smaller Ni3+ (0.56 Å) and Co3+ (0.545 Å) in the host lattice with larger Sn4+ (0.69 Å) [37]. In contrast, the (003) peaks of the NC82_Sn5% samples synthesized at temperatures above 650°C gradually shifted to a higher angle owing to the decreased solubility of Sn in NC82, and they were higher than that of NC82, which strongly confirms the successful doping of Sn.
To further evaluate the structural changes with increasing temperature, Rietveld refinement of the XRD patterns of the NC82 and NC82_Sn5% powders at various synthesis temperatures was conducted (Fig. 2b). As the synthesis temperature increased from 600 to 800°C, Sn content in the NC82_Sn5% host structure decreased from 4.96±0.27% to 1.10±0.26%, and the a- and c-axis lattice parameters decreased, which confirm that larger Sn4+ ions separated from the host structure. The Sn content decreased at 800°C, whereas the lattice parameters and degree of Li/Ni mixing unexpectedly increased. Although Sn separated from the host material owing to its lower solubility, the inherent structural instability of high-Ni cathodes at high temperatures can cause a significant degree of Li/Ni mixing. Based on these results, the optimum synthesis temperature for NC82 with or without Sn is 750°C.
The morphologies of NC82 and NC82_Sn5% were investigated using SEM, STEM, and EDS mapping. NC82 with or without Sn at 750°C was hundreds of nanometers in size with a plate-like morphology, as shown in Fig. 3a,b. To gain an in-depth understanding of the Sn distribution as a function of temperature, NC82_Sn5% samples synthesized at two different temperatures (600 and 750°C) were further characterized using STEM-HAADF coupled with EDS. Fig. 3c,d show the EDS elemental mapping of NC82_Sn5% at 600 and 750°C. The elemental mapping of Sn in NC82_Sn5% at 600°C clearly exhibited a uniform distribution of Sn, which covered the entire particle, demonstrating the successful doping of Sn in NC82. However, for NC82_Sn5% at 750°C, a thin layer (~5 nm) of Li2SnO3 was observed at the cathode surface and a uniform distribution of Sn was observed in the bulk, indicating the simultaneous surface coating of Sn in high-Ni cathode materials. These results are consistent with the XRD structural analysis results.
The electrochemical performances of NC82 and NC82_Sn5% were evaluated in a coin-type half-cell with a lithium-metal anode as the reference and counter electrodes at 30°C. The initial galvanostatic charge and discharge voltage profiles of the formation cycle for the two samples in the half cells at a rate of C/10 in the range of 3.0–4.4 V (vs. Li/Li+) are shown in Fig. 4a. NC82 and NC82_Sn5% exhibited discharge capacities of 201 and 190 mA h g−1, respectively, with initial Coulombic efficiencies (ICEs) of 92.3% and 91.5%, respectively. Additionally, the rate performances of the two samples were evaluated at various current rates from C/10 to 10 C in the range of 3.0–4.4 V (vs. Li/Li+), as shown in Fig. 4b. NC82_Sn5% exhibited a significantly better rate performance than NC82. Fig. 4c,d show the capacity retention and Coulombic efficiency (CE) of the two samples at a rate of C/2 for 100 cycles at 30°C. The NC82_Sn5% cathode exhibited a better capacity retention (70.9%) than NC82_Sn5% (64.8%), and the CE of NC82_Sn5% was higher than that of NC82 over the 100 cycles. In addition, the cycle performance of NC82_Sn5% demonstrates superior capacity retention compared to that of NC82 when subjected to an elevated temperature of 60°C (Fig. 4e). These results are attributed to the Li2SnO3 coating layer that prevents electrolyte decomposition by minimizing the contact between the Ni4+ in the cathode electrode and the electrolyte, resulting in increased interfacial resistance between the cathode and the electrolyte compared to NC82.
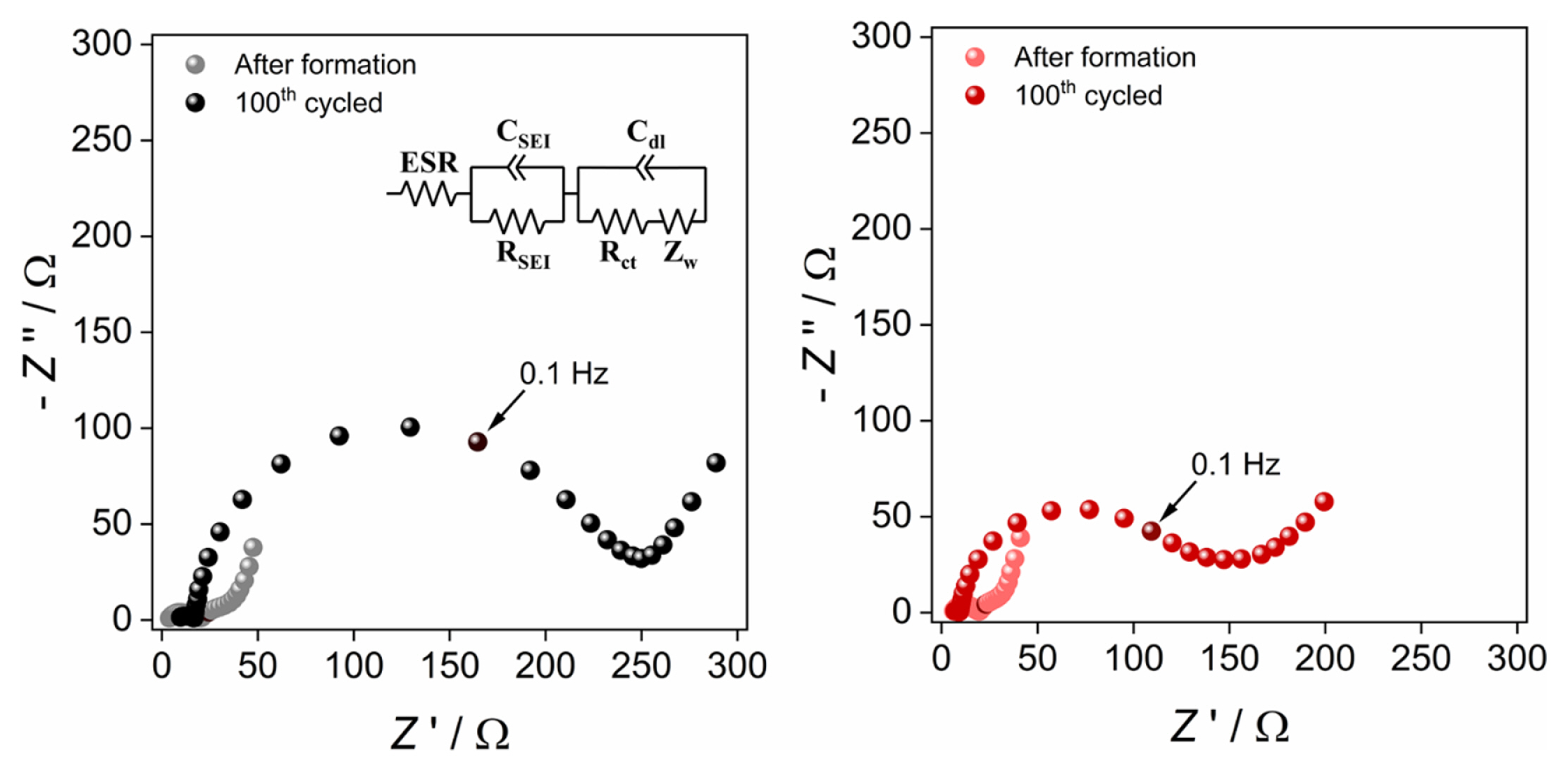
Because the introduction of a Li2SnO3 layer on the cathode surface can increase the interfacial stability of the NC82 electrode by reducing anodic side reactions, electrochemical impedance spectroscopy was used to measure the change in charge transfer resistance after cycling the NC82 electrode (EIS). Fig. 5 depicts Nyquist plots of NC82 and NC82_Sn5% electrodes after formation and after 100 cycles. In Nyquist plots, the recorded resistance value at 0.1 Hz of frequency is indicated since charge transfer in NCM materials is generally observed at frequencies less than 0.1 Hz [38]. Using EIS measurement in a Li/NC82 cell, a significant increase in total resistance is detected, accompanied by an increase in charge transfer resistance. Meanwhile, because the anodic electrolyte decomposition on the surface of NC82 is suppressed by the Li2SnO3 functional layer, the total resistance development during cycle at the Li/ NC82_Sn5% cell is highly decreased. Consequently, the cycle performance of the NC82 is greatly improved due to the lower charge transfer resistance growth at NC82_Sn5% by Li2SnO3 layer.
Moreover, as mentioned previously, the impact of structural changes on the high-Ni cathode should be considered in addition to the surface stability. Fig. 6 shows the degree of Li/Ni mixing of the two electrodes after 100 cycles determined by ex situ XRD and Rietveld refinement analyses. The difference in the degree of Li/Ni mixing between the two samples was insignificant before and after cycling. Therefore, the impact of the structural changes between the two NC82 samples is insignificant, and the surface β-Li2SnO3 layer contributed to the improved cycle performance.
In summary, this study demonstrated a one-step coating method for high-Ni layered oxide cathode materials based on the solubility limits of Sn in LiNi0.8Co0.2O2 as a function of temperature. Sn was doped into NC82 at 600°C, and its solubility decreased with increasing temperature. Consequently, Sn separated from the host material and a β-Li2SnO3 layer was formed on the surface of the high-Ni cathode. The transition of Sn with respect to temperature was systematically investigated using XRD and STEM coupled with EDS. The electrochemical performances of NC82 and NC82_Sn5% were compared in the range of 3.0–4.4 V (vs. Li/Li+). NC82_Sn5% exhibited better rate and cycle performances than bare NC82. Using a combination of structural and electrochemical analyses, including CE, EIS, and the degree of Li/Ni mixing, the β-Li2SnO3 layer of NC82_Sn5% prevented surface electrolyte decomposition during cycling, thereby improving the cycle performance of NC82_Sn5%. Therefore, we strongly believe that one-step coating engineering by thermal phase segregation is a prospective method for interphase stabilization to prevent unwanted surface side reactions with electrolytes in LIBs.
Acknowledgements
We acknowledge the support from a HRD Program for Industrial Innovation (P0012748) by Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE), Ministry of Trade, Industry & Energy/Korea Evaluation Institute of Industrial Technology (MOTIE/KEIT) (Nos. 20010900) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2022M3J1A1085408). This research was also supported by “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE)(2022RIS-005).
Fig. 1
Schematic diagram for the one-pot synthesis of β-Li2SnO3 layer coated NC82 with increasing temperature.
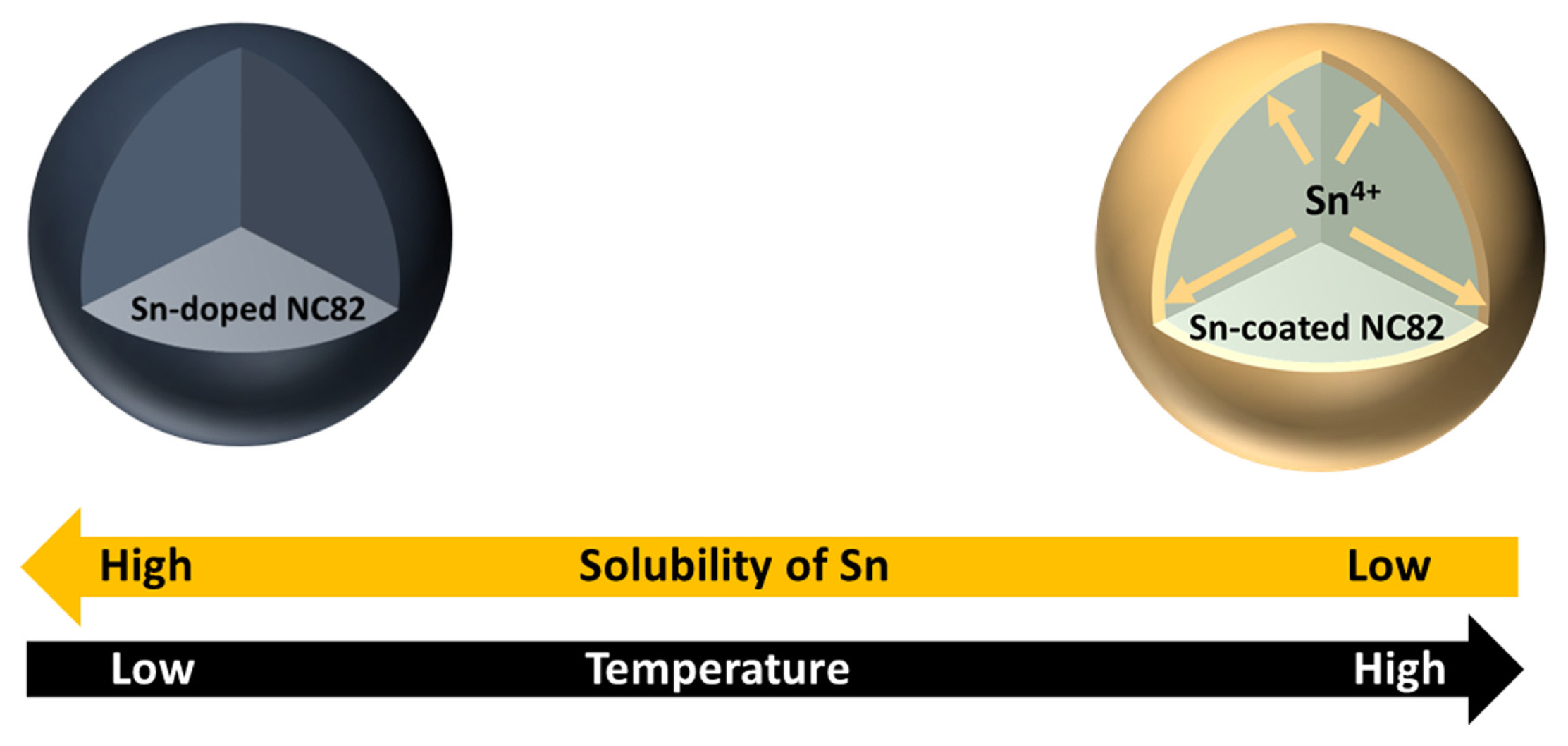
Fig. 2
(a) XRD patterns of NC82 and NC82_Sn5% as a function of synthesis temperature and (b) the Rietveld refinement results of the XRD patterns.

Fig. 3
SEM images of (a) NC82 and (b) NC82_Sn5%. STEM images and the corresponding EDS elemental maps of Ni (green), Co (yellow), and Sn (red) for NC82_Sn5% at (c) 600°C and (d) 750°C.
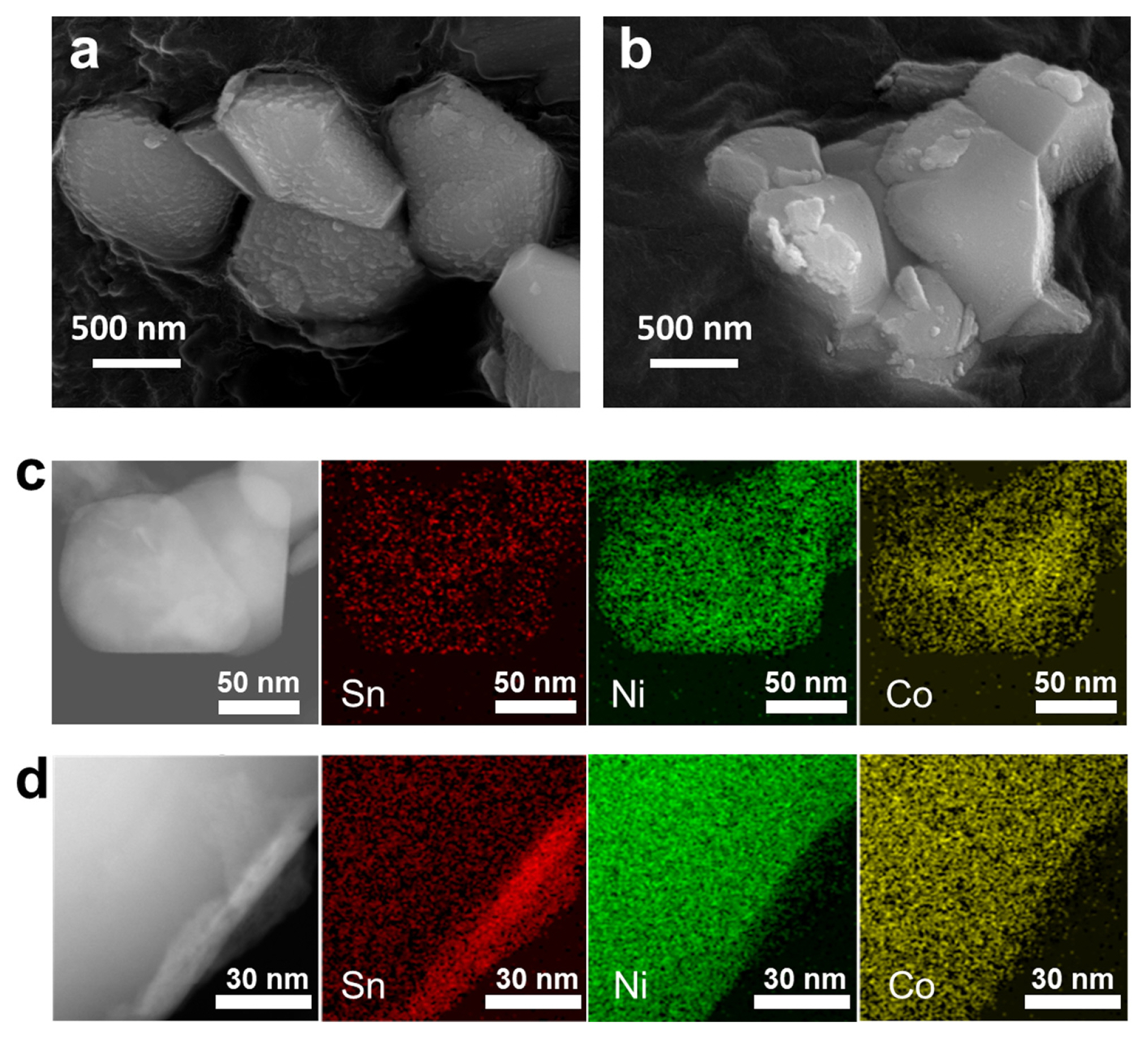
Fig. 4
Electrochemical characteristics of NC82 and NC82_Sn5%: (a) voltage profiles of the formation cycle at a rate of C/ 10, (b) rate performance, (c) cycle performance, and (d) Coulombic efficiency at a rate of 1 C in the range of 3.0–4.4 V (vs. Li/Li+) at 30°C; (e) cycle performances at 60°C.

References
[1] F. Hasan, J. Kim, H. Song, S. H. Lee, J. H. Sung, J. Kim and H. D. Yoo, J. Electrochem. Sci. Technol, 2020, 11(4), 352–360.
[4] J. H. Yang, S. J. Hwang, S. K. Chun and K. J. Kim, J. Electrochem. Sci. Technol, 2022, 13(2), 208–212.


[7] J. Kim, S. Lee, K. W. Kim, J. Son and J. Mun, J. Electrochem. Sci. Technol, 2021, 12(4), 424–430.


[8] H. Kim, T. H. Kim, W. Kim, S. S. Park and G. Jeong, ACS Appl. Mater. Interfaces, 2023, 15(7), 9212–9220.


[9] M. A. Lee, J. B. Lee, J.-N. Lee, S.-G. Woo, J. Yu and H. Kim, J. Electroanal. Chem, 2022, 905, 115900.

[12] H. Kim, G. Jeong, H. J. Leem, M. A. Lee, J.-N. Lee, S.-G. Woo and J. Yu, J. Mater. Chem. A, 2022, 10, 17659–17667.

[13] S. W. Doo, K. Kim, H. Kim, S. Lee, S. H. Choi and K. T. Lee, J. Electrochem. Soc, 2021, 168, 100529.


[14] Y. Kim, D. Koo, S. Ha, S. C. Jung, T. Yim, H. Kim, S. K. Oh, D.-M. Kim, A. Choi, Y. Kang, K. H. Ryu, M. Jang, Y.-K. Han, S. M. Oh and K. T. Lee, ACS Nano, 2018, 12(5), 4419–4430.

[15] J. Jang, Y. Kim, O. B. Chae, T. Yoon, S.-M. Kim, H. Kim, H. Park, J. H. Ryu and S. M. Oh, Angew. Chem., Int. Ed, 2014, 53(40), 10654–10657.

[16] H. Kim, S. K. Oh, J. Lee, S. W. Doo, Y. Kim and K. T. Lee, Electrochim. Acta, 2021, 370, 137743.

[17] Y. Kim, H. Park, A. Dolocan, J. H. Warner and A. Manthiram, ACS Appl. Mater. Interfaces, 2021, 13(23), 27096–27105.

[18] S. Jeong, K. Choi, V.-C. Ho, J. Cho, J.-S. Bae, S. C. Nam, T. Yim and J. Mun, Chem. Eng. J, 2022, 434, 134577.

[19] S. Jeong, S. Young Park, B. So, K. Tae Lee, Y. D. Park and J. Mun, Chem. Eng. J, 2022, 448, 137654.

[20] S. Jeong, J. H. Park, S. Y. Park, J. Kim, K. T. Lee, Y. D. Park and J. Mun, ACS Appl. Mater. Interfaces, 2021, 13(19), 22475–22484.

[22] H.-H. Ryu, N.-Y. Park, T.-C. Noh, G.-C. Kang, F. Maglia, S.-J. Kim, C. S. Yoon and Y.-K. Sun, ACS Energy Lett, 2021, 6(1), 216–223.

[23] H.-H. Ryu, N.-Y. Park, J. H. Seo, Y.-S. Yu, M. Sharma, R. Mücke, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, Mater. Today, 2020, 36, 73–82.

[24] U.-H. Kim, L.-Y. Kuo, P. Kaghazchi, C. S. Yoon and Y.-K. Sun, ACS Energy Lett, 2019, 4(2), 576–582.


[25] S. Jamil, G. Wang, L. Yang, X. Xie, S. Cao, H. Liu, B. Chang and X. Wang, J. Mater. Chem. A, 2020, 8, 21306–21316.

[26] H.-H. Ryu, H.-W. Lim, G.-C. Kang, N.-Y. Park and Y.-K. Sun, ACS Energy Lett, 2023, 8(3), 1354–1361.


[28] F. Wu, N. Liu, L. Chen, Y. Su, G. Tan, L. Bao, Q. Zhang, Y. Lu, J. Wang, S. Chen and J. Tan, Nano Energy, 2019, 59, 50–57.

[30] F. Schipper, E. M. Erickson, C. Erk, J.-Y. Shin, F. F. Chesneau and D. Aurbach, J. Electrochem. Soc, 2017, 164, A6220.

[31] F. Wu, S. Fang, M. Kuenzel, A. Mullaliu, J.-K. Kim, X. Gao, T. Diemant, G.-T. Kim and S. Passerini, Joule, 2021, 5(8), 2177–2194.

[32] Y. Kim, H. Park, K. Shin, G. Henkelman, J. H. Warner and A. Manthiram, Adv. Energy Mater, 2021, 11(38), 2101112.


[33] V.-C. Ho, H. An, M. Hong, S. Lee, J. Kim, M. B. Park and J. Mun, Energy Technol, 2021, 9(2), 2000800.


[34] D. Becker, M. Börner, R. Nölle, M. Diehl, S. Klein, U. Rodehorst, R. Schmuch, M. Winter and T. Placke, ACS Appl. Mater. Interfaces, 2019, 11(20), 18404–18414.

[35] D. Kitsche, F. Strauss, Y. Tang, N. Bartnick, A.-Y. Kim, Y. Ma, C. Kübel, J. Janek and T. Brezesinski, Batteries & Supercaps, 2022, 5(6), e202100397.
- TOOLS