 |
 |
- Search
| J. Electrochem. Sci. Technol > Volume 10(2); 2019 > Article |
|
Abstract
An electrochemical detection method for capsaicin has been developed using ionic liquid (IL) doped graphene-titania-Nafion composite-modified electrode. The combination of IL (1-hexyl-3-methylimidazolium with hexafluorophosphate counter ion) in the composite-modified electrode resulted in a significantly increased electrochemical response for capsaicin compared to that obtained at the corresponding electrode without IL. The increased electrochemical signal could be ascribed to the decreased electron transfer resistance through the composite film and also to the effective accumulation of capsaicin on the electrode surface due to π-π interaction of the imidazole groups of IL with the aromatic rings of capsaicin. The present IL composite-modified electrode can detect capsaicin with a concentration range from 3.0 × 10−8 M to 1.0 × 10−5 M with a detection limit of 3.17 × 10−9 M (S/N = 3). The present sensor showed good reproducibility (RSD = 3.2%).
Pepper is one of the oldest plants in the world. It has been used mainly as a spice due to its pungent taste. In particular, capsaicinoids are compounds that are responsible for the pungent taste in pepper fruits and their products [1]. Capsaicin and dihydrocapsaicin are the most abundant capsaicinoids in peppers, accounting for about 90% of the total 11 capsaicinoids [2,3]. Pungency is related to the concentration of capsaicin in the pepper. Many health benefits related to capsaicin are known [4,5]. For example, capsaicin is analgesic for inflammation [6] and also anticarcinogenic [7]. It has also degrading effects for cholesterol and obesity [8–10], and are effective antioxidants [11,12]. However, high levels of capsaicin in human body have a negative impact on health. Therefore, the development of a simple detection method for capsaicin in food and pharmaceuticals is very important.
To date, a number of detection methods for capsaicin have been reported. Typically, the pungent taste is measured through Scoville organoleptic test, in which a trained volunteer detects the level of pungency by tasting a pepper extract solution diluted in sugar water [13]. In addition, high performance liquid chromatography [14], gas chromatography-mass spectrometry [15], HPLC-UV [16], HPLC-fluorescence [17], colorimetry [18,19] and capillary electrophoresis [20] were employed for the determination of capsaicin in food and pharmaceuticals. Despite the good analytical performance, such analytical methods are suffering from requirements for complex sample preparation steps, expensive tools, long analysis times and skilled technicians. Therefore, electrochemical methods for capsaicin have attracted strong attention because they are fundamentally simple and can be fabricated as a portable device. Since Compton and his coworkers published the first electrochemical detection of capsaicin [21], a number of electrochemical methods have been reported over the last 10 years. For example, various composite-based electrodes such as gold nanoparticles (NPs)/multi-walled carbon nanotube [22], Ag/Ag2O NPs/reduced graphene oxide [23], ruthenium NPs/carbon nanotubes [24], and poly(sodium 4-styrenesulfonate)/graphite/screen printed electrode [25] were reported. Although the previous reports have demonstrated their good analytical performance for capsaicin detection, further research and development is still needed to apply an electrochemical capsaicin sensor to the real-world field analysis.
Previously, we reported the graphene-doped solgel titania-Nafion composite-modified electrode for the determination of capsaicin in real samples [26]. In the present study, an ionic liquid (IL) composite-modified electrode was developed and characterized for the determination of capsaicin in food sample. To the best of our knowledge, IL has not been used for the preparation of composite-modified electrode for capsaicin. ILs have been widely applied to electrochemical sensors due to their low volatility, thermal stability, high electrical conductivity, wide electrochemical potential range, and stability [27,28]. ILs can be used as a modifier for the modified electrodes as well as the electrolytes [29], and effective dispersion of the graphene is possible through ‘cation-pi’ interactions with the use of ILs [30,31]. Up to now, various kinds of electrochemical sensors have been manufactured based on different ILs [32–36]. In the present work, the fabrication and characterization of the IL-composite modified electrode will be reported and their analytical performance will be compared to that obtained at the corresponding electrode without IL.
Cyclic voltammetry (CV) was performed with an EG&G 263A potentiostat (Oak Ridge, TN, USA). Electrochemical impedance spectroscopy (EIS) experiments were carried out with frequency response detector (Model 1025, Oak Ridge, TN, USA). Three-electrode system was consisted of platinum wire as counter electrode, working GC electrode (area = 0.071 cm2) and Ag/AgCl (3 M NaCl) reference electrode in 10 mL cell. Electrochemical impedance spectroscopy (EIS) experiments were performed in 5.0 mM K3Fe(CN)6/K4Fe(CN)6 (1:1, v/v) solution dissolved in 50 mM phosphate buffer at pH 7.0. Scanning electron microscopy (SEM) images were obtained using field emission SEM (JSM-7100F, JEOL Ltd. Japan). Energy dispersive spectroscopy (EDS) measurements were performed using electron probe of JSM-7100F (JEOL Ltd. Japan).
Capsaicin, titanium(IV) isopropoxide (99.999% trace metals basis), Nafion® perfluorinated resin solution (5 wt.% in lower aliphatic alcohol and water, contains 15–20% water), 1-ethyl-3-methylimidazolium hexafluorophosphate, 1-butyl-3-methylimidazolium hexafluorophosphate, 1-hexyl-3-methylimidazolium hexafluoro-phosphate, 1,3-dimethoxy-2-methylimidazolium hexafluorophosphate, 2-propanol, ethanol, acetic acid, boric acid, phosphoric acid, sodium hydroxide, and hydrochloric acid were purchased from Sigma-Aldrich. Reduced graphene oxide powder was purchased from Graphene-Supermarket (Graphene Laboratories, NY, USA). The stock solution of capsaicin (1.0 mM) was prepared by dissolving in ethanol. Working solution was prepared by diluting the stock solution with 0.04 M Britton-Robinson buffer solutions. Britton-Robinson (BR) buffer solutions were prepared by mixing solutions of acetic acid, boric acid and phosphoric acid and then adjusting the pH with HCl or NaOH [37,38].
The glassy carbon electrode (GCE) modified with IL composite has been fabricated in a multistep sequence. First, a bare GCE was cleaned and then titania-Nafion composite solution was prepared according to the previous report [26]. Graphene and ILs were then dispersed in the composite solution, and the mixture was placed in an ultrasonic bath for 60 min. Finally, a 2 μL aliquot of the resulting IL composite solution was hand-casted on the surface of a pre-treated GCE using a micro-injector. The modified GCE was dried for 20 min at room temperature and was immersed in buffer solution in order to swell the composite.
The as-prepared IL composite-modified GCE was immersed in the capsaicin solution and stirred for 10 min. CV and EIS experiments were performed according to the previous report [26].
In this study, the reduced graphene oxide was easily dispersed in the IL-titania-Nafion composite solution, which is similar to the case of single-walled carbon nanotube in our previous study [36]. The IL composite-modified electrodes were prepared by simply hand-casting a small aliquot of composite solution onto the surface of a pre-cleaned GCE.
SEM images were gained to study the morphology of the composite. As shown in Fig. 1, the graphenetitania-Nafion composite incorporated with 1-hexyl-3-methylimidazolium hexafluorophosphate (HMIHP) shows three-dimensional micro-pore structure of composite film. Thus, the highly porous nature enables the faster diffusion of capsaicin through the interconnected porous channels of the composite. In addition, the EDS mapping image of the IL composite film shows even distribution of fluorine (A) and titanium (B) atoms as shown in Fig. 2. The SEM images indicates that IL containing counter anion (hexafluorophosphate), Nafion (fluorocarbon backbone) and sol-gel titania (TiO2) are well mixed together in the composite.
The electrochemical behaviour of the IL composite-modified electrodes were examined using CV and EIS. Fig. 3 shows the Nyquist plots of EIS obtained at different modified electrodes. The measured charge transfer resistances were 9.31 × 102 Ω for a bare GCE and 2.76 × 104 Ω for the titania-Nafion composite-modified GCE. Because of the electrostatic repulsion between the anionic redox probe and the anionic titania-Nafion composite films, charge transfer resistance value of titania-Nafion composite-modified GCE was larger than that of bare GCE. However, the addition of IL to the negatively charged titania-Nafion composite led to the decreased resistance of 4.87 × 103 Ω. The results indicate that IL has the positive effect of increasing the conductivity as well as decreasing the overall negative charge of the titania-Nafion composite. Finally, the inclusion of graphene to the composite resulted in the additional decrease in charge transfer resistance to 7.05 × 102 Ω due to the excellent electron transfer capability and conductivity of graphene in the composite.
A linear sweep voltammogram with different composite-modified electrodes were obtained for 0.5 μM capsaicin in 0.04 M BR buffer (pH 1.0). As shown in Fig. 4, the smallest oxidation peak current of 1.54 μA was observed with peak potential at about 0.75 V in titania-Nafion composite-modified GCE (a). When the GCE was modified with an IL-titania-Nafion composite (b), the oxidation peak current (11.6 μA) was about 7.5 times greater than that without IL. However, it is still smaller than the oxidation peak current (59.8 μA) at the GCE modified with a graphene-titania-Nafion composite (c). Finally, when the GCE was modified with an IL-graphene-titania-Nafion composite (d), the greatest peak oxidation current of 101 μA was observed for 0.5 μM capsaicin.
Although 1-hexyl-3-methylimidazolium hexafluorophosphate is most effective for the construction of composite-modified electrode in the present study as shown in Fig. 5, 1-butyl-3-methylimidazolium, 1-ethyl-3-methylimidazolium, 1,3-dimethoxy-2-methy imidazolium and 1-benzyl-3-methylimidazolium hexafluorophosphate can also be used. The chemical structures of the ionic liquids used, as well as the reaction mechanism, are shown in Scheme 1. Regardless of the type of the ILs, the incorporation of IL in the composite resulted in the enhancement in the electrochemical signal for capsaicin. Each ionic liquid is known to exhibit different electrocatalytic properties when combined with graphene due to different interactions with graphene for each IL [30], thus the different IL composite-modified resulted in slightly different electrochemical responses. As a result, HMIHP IL was used for all subsequent experiments since it produced the largest electrochemical signal for capsaicin.
The accumulation time under the open-circuit potential had an influence on the amount of capsaicin adsorbed on the surface of the modified GCE, thus affecting the oxidation current. As shown in Fig. 6, the oxidation current was shown to increase with accumulation time. When comparing the measured signal values at 3 minutes of accumulation, the current signal for capsaicin at the composite-modified GCE without IL was almost negligible, whereas a relatively large oxidation current of 2.16 μA was observed at IL composite-modified GCE. The current at the IL composite-modified GCE no longer increased even when the accumulation time was increased to more than 6 minutes, indicating that capsaicin is saturated on the surface of composite-modified GCE. In addition, it was observed that the time required for the saturation of capsaicin on the electrode surface is relatively faster at the IL composite-modified GCE than at the composite-modified GCE without the IL (~ 9 min). This result clearly indicates that the incorporation of ILs into the composite leads to increased electron transfer ability of the electrode, as well as effective accumulation of capsaicin on the electrode surface through π-π interaction between the imidazole groups of ILs and the aromatic rings of capsaicin. Therefore, a highly sensitive electrochemical sensor for capsaicin was achieved through the incorporation of IL, along with electrocatalytically active graphene, in the titania-Nafion composite film.
The amount of IL incorporated into the composite was shown to affect the electrochemical signal of capsaicin. Fig. 7 shows the change in the peak current for 100 μM capsaicin according to the various concentrations of HMIHP IL in the composite. Composite with 50 mM IL displayed a significant enhancement of the oxidation current due to excellent conductivity of IL. However, the signal value of capsaicin decreased with increasing concentration of IL when the concentration exceeded 50 mM. This may be due to the saturation of IL in the composite as well as decreased stability of the composite with excessive amount of IL [36]. Further, excessive amount of IL thickens the film formed on the electrode surface, which hinders the electron transfer [39]. Thus, 50 mM was used for all subsequent experiments.
The amount of graphene incorporated into the composite had a significant effect on the electrochemical response of capsaicin. As seen in Fig. 8, the oxidation current of capsaicin increased with increasing the graphene content in the range from 0.75 mg/mL to 2.0 mg/mL. This result suggests improved charge transfer on the electrode surface as well as the accumulation of capsaicin. However, further increasing the amount of graphene in the composite to more than 2.0 mg/mL led to a decrease in the signal value for capsaicin since the graphene is no longer uniformly dispersed in the composite. These results show that more graphene (2.0 mg/mL) can be dispersed in IL modified composite compared with the previously reported titania-Nafion composite without IL (1.5 mg/mL) [26]. This result clearly indicates that IL has solubility for graphene.
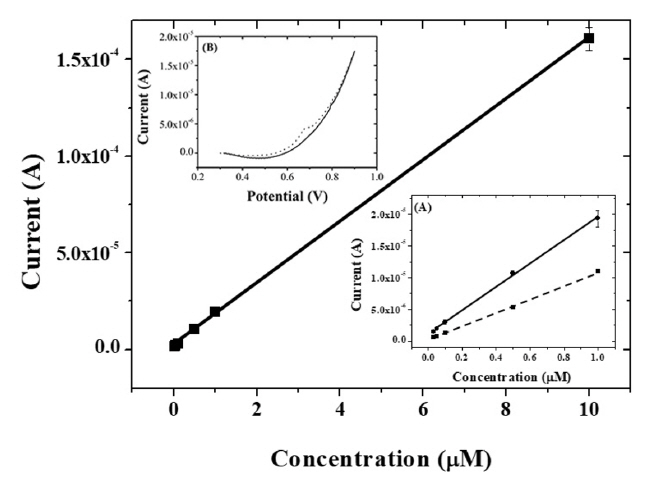
Under optimized conditions, calibration curves (peak current vs. concentration of capsaicin) were constructed with the present IL composite-modified GCE using linear sweep voltammetry at a scan rate of 100 mv/s after accumulation of capsaicin for 10 min. As shown in Fig. 9, the present composite-modified electrode had a linear response from 0.03 μM to 10 μM of capsaicin. The regression equation was estimated to be ipa1 (A) = 1.94 × 10−5 C (μM) + 8.76 × 10−7 (R2 = 0.996) with a detection limit of 3.17 × 10−9 M (S/N = 3). As shown in Table 1, the dynamic range and detection limit obtained at the present IL composite-modified GCE were superior to other modified electrodes in previously reported literatures.
Sensor-to-sensor reproducibility of the present composite-modified electrode for capsaicin detection was investigated using five freshly prepared composite-modified electrodes. The reproducibility for the fabrication for the fabrication of the composite-modified electrode was relatively good (RSD= 3.2%). A recovery test has been performed in a solution of capsaicin extracted from Korean hot pepper (Chungyang pepper) samples. Capsaicin was extracted with a method similar to that of a previous report [40]. For recovery test, capsaicin solutions with known concentrations were spiked into the prepared pepper solution. The results showed good recoveries, as listed in Table 2. These results clearly demonstrate that the present IL composite-modified electrode can be effectively applied to the capsaicin analysis in real samples.
The present study clearly demonstrated that the IL can be effectively used for the fabrication of a sensitive electrochemical capsaicin sensor. Due to the high ionic conductivity of ILs, the addition of ILs in the composite has decreased the electron transfer resistance in the composite. In addition, IL in the composite enables the rapid and effective accumulation of capsaicin on the composite-modified electrode surface due to π-π interaction between aromatic rings of capsaicin and imidazole groups of ionic liquids, thus leading to a significant increase in electrochemical response as similar to the case of bisphenol A detection [36]. Since the proposed electrochemical sensor has superior analytical performance for capsaicin to the previous reports, it is expected to be able to quantify pungency through direct detection of capsaicin in food and pharmaceuticals, and used as an alternative to the Scoville organoleptic test.
Acknowledgement
Financial support for this work has been provided by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2015R1D1A1A01058396).
Fig. 1
Scanning electron microscopy image of the ionic liquid-graphene-titania-Nafion composite film. Scale bar: 500 nm.
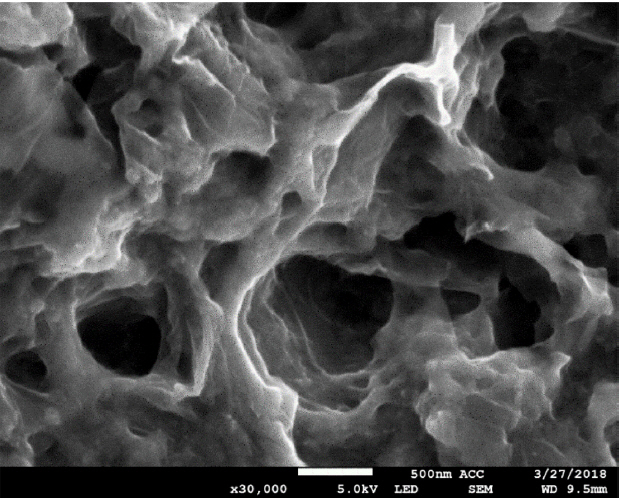
Fig. 2
Energy-dispersive X-ray spectroscopy mapping image of the ionic liquid-graphene-titania-Nafion composite. (A) Fluorine (B) Titanium.
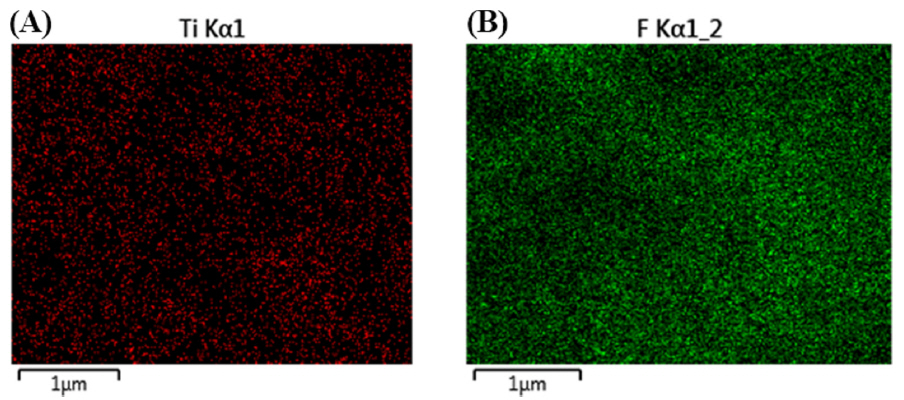
Fig. 3
Nyquist plots for the impedance measurements in the presence of 5.0 mM K3Fe(CN)6/K4Fe(CN)6 in 0.05 M phosphate buffer (pH 7.0) at a bare GCE (□), titania-Nafion (○), ionic liquid-titania-Nafion (♦), ionic liquid-graphene-titania-Nafion (▲) composite modified glassy carbon electrode.
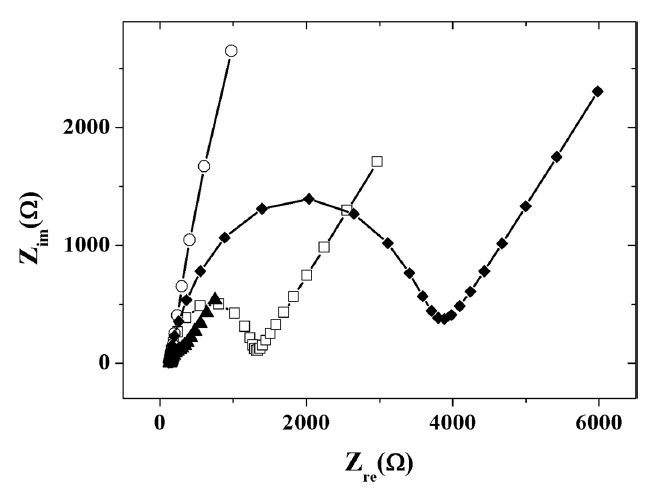
Fig. 4
Linear sweep voltammograms of 0.5 μM capsaicin at a titania-Nafion (a), ionic liquid-titania-Nafion (b), graphene-titania-Nafion (c) and ionic liquid-graphene-titania-Nafion (d) composite modified glassy carbon electrode in 0.04 M Britton-Robinson buffer (pH 1.0) at a scan rate of 100 mV/s.
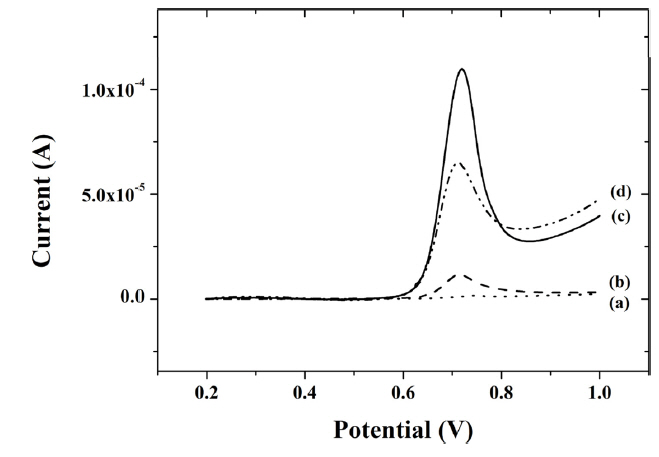
Fig. 5
Oxidation current of 10 μM capsaicin obtained at glassy carbon electrodes modified with titania-Nafion composites incorporated with 1-butyl-3-methylimidazolium (A), 1-hexyl-3-methylimidazolium (B), 1-ethyl-3-methylimidazolium (C), 1,3-dimethoxy-2-methylimidazolium (D) and 1-benzyl-3-methylimidazolium (E) hexafluorophosphate in 0.05 M pH 7.0 phosphate buffer at a scan rate was 100 mV/s.
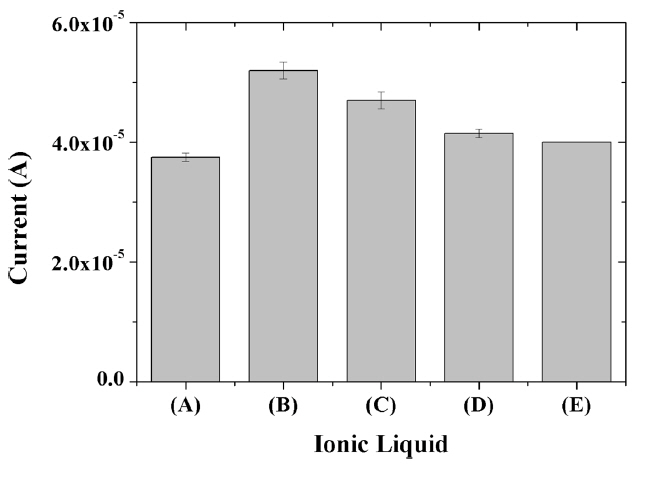
Fig. 6
Effect of the accumulation time on the oxidation peak current of 0.1 μM capsaicin at the graphene-titania-Nafion (dashed line) and ionic liquid-graphene-titania-Nafion (solid line) composite-modified electrode in 0.04 M Britton-Robinson buffer (pH 1.0) at a scan rate of 100 mV/s.
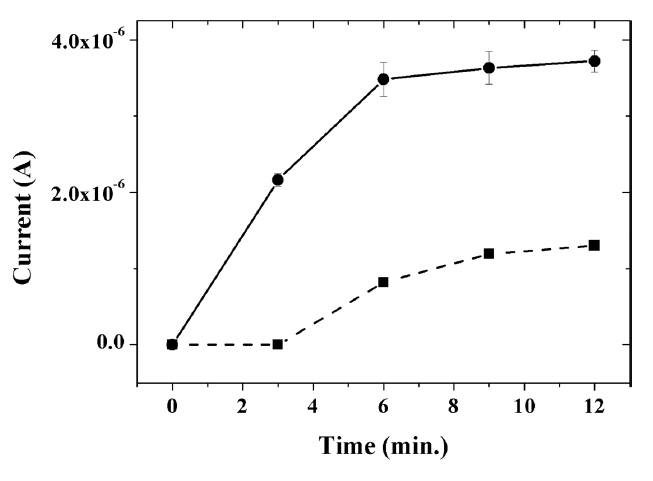
Fig. 7
Effect of concentration of ionic liquid on the oxidation peak current of 100 μM capsaicin at the composite-modified electrode in 0.04 M Britton-Robinson buffer (pH 1.0) at a scan rate of 100 mV/s.
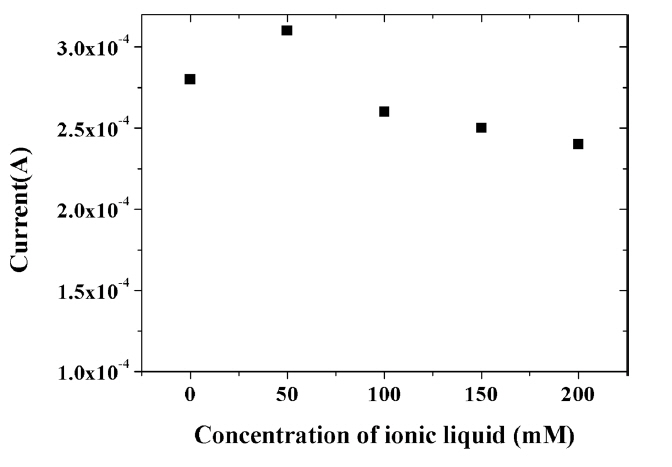
Fig. 8
Effect of amount of graphene on the oxidation peak current of 100 μM capsaicin at the composite-modified electrode in 0.04 M Britton-Robinson buffer (pH 1.0) at a scan rate of 100 mV/s.
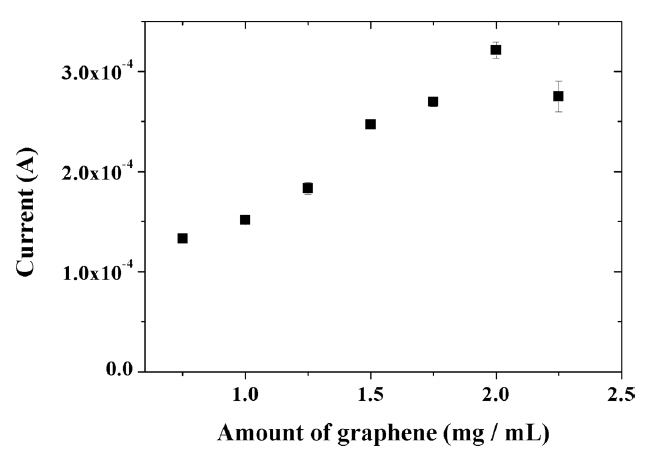
Fig. 9
Calibration curves for capsaicin obtained at the composite-modified electrode in the concentration of 10 μM, 1 μM, 0.5 μM, 0.1 μM, 0.05 μM, and 0.03 μM. Insert (A): calibration curves for capsaicin obtained at ionic liquid-graphene-titania-Nafion (solid line) and graphene-titania-Nafion (dashed line) composite-modified electrode in the concentration range from 0.03 μM to 1.0 μM. (B): Linear sweep voltammograms of buffer (solid line) and 0.03 μM capsaicin (dotted line) at ionic liquid-graphene-titania-Nafion composite.
Scheme 1
Reaction mechanism of the oxidation of capsaicin, and the chemical structures of the ionic liquids used: 1-butyl-3-methylimidazolium (A), 1-hexyl-3-methylimidazolium (B), 1-ethyl-3-methylimidazolium (C), 1,3-dimethoxy-2-methylimidazolium (D) and 1-benzyl-3-methylimidazolium (E) hexafluorophosphate.
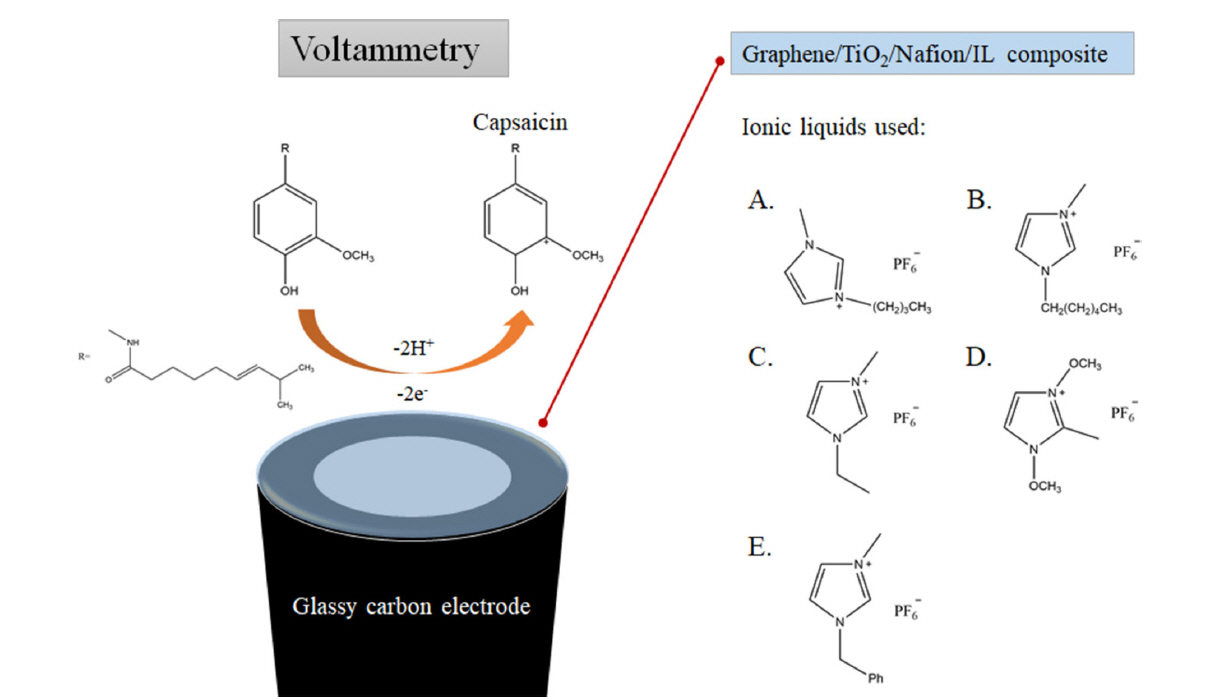
Table 1
Comparison of the present capsaicin sensor with different reported methods.
| Composite-modified electrode | Dynamic range (μM) | Detection limit (μM) | Reference |
|---|---|---|---|
| CNT-SPE | 0.5–35.0 | 0.45 | [21] |
| AuNPs-MWCNT-GCE | 0.15–35.0 | 0.0027 | [22] |
| Ag/Ag2O-PSS-rGO-SPE | 1.0–60 | 0.4 | [23] |
| RuNPs-CNTs-GCE | 0.01–0.41 | 0.0025 | [24] |
| PSS-graphite-SPE | 0.3–70 | 0.1 | [25] |
| rGO-titania-Nafion-GCE | 0.03–10 | 0.0086 | [26] |
| Ionic liquid-rGO-titania-Nafion-GCE | 0.03–10 | 0.0032 | This work |
4. References
[4] MJ. Caterina, MA. Schumacher, M. Tominaga, TA. Rosen, JD. Levine and D. Julius, Nature, 1997, 389(6653), 816.

[7] C. Ganguly, Asian Pacific Journal of Cancer Prevention, 2010, 11(1), 25–8.
[8] JA. Negulesco, RM. Young and P. Ki, Artery, 1985, 12(5), 301–311.
[12] A. Rosa, M. Deiana, V. Casu, S. Paccagnini, G. Appendino, M. Ballero and MA. Dessi, J Agric Food Chem, 2002, 50(25), 7396–7401.

[14] SH. Choi, BS. Suh, E. Kozukue, N. Kozukue, CE. Levin and M. Friedman, J Agric Food Chem, 2006, 54(24), 9024–9031.

[15] A. Peña-Alvarez, E. Ramírez-Maya and L. Alvarado-Suárez, J Chromatogr A, 2009, 1216(14), 2843–2847.

[20] LH. Liu, XG. Chen, JL. Liu, XX. Deng, WJ. Duan and SY. Tan, Food Chem, 2010, 119(3), 1228–1232.

[22] T. Mpanza, MI. Sabela, SS. Mathenjwa, S. Kanchi and K. Bisetty, Anal Lett, 2014, 47(17), 2813–2828.

[30] T. Fukushima, A. Kosaka, Y. Ishimura, T. Yamamoto, T. Takigawa, N. Ishii and T. Aida, Science, 2003, 300(5628), 2072–2074.

[31] A. Abo-Hamad, MA. AlSaadi, M. Hayyan, I. Juneidi and MA. Hashim, Electrochim Acta, 2016, 193, 321–343.

[32] X. Niu, W. Yang, J. Ren, H. Guo, S. Long, J. Chen and J. Gao, Electrochim Acta, 2012, 80, 346–353.

[33] S. Hu, YH. Wang, XZ. Wang, L. Xu, J. Xiang and W. Sun, Sens Actuators B Chem, 2012, 168, 27–33.

[34] C. Shan, H. Yang, D. Han, Q. Zhang, A. Ivaska and L. Niu, Biosens Bioelectron, 2010, 25(6), 1504–1508.

[35] Q. Zhang, S. Wu, L. Zhang, J. Lu, F. Verproot, Y. Liu, Z. Xing, J. Li and XM. Song, Biosens Bioelectron, 2011, 26(5), 2632–2637.

- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 6,688 View
- 110 Download
- Related articles in J. Electrochem. Sci. Technol
-
Further Electrochemical Degradation of Real Textile Effluent Using PbO2 Electrode2021 May;12(2)





