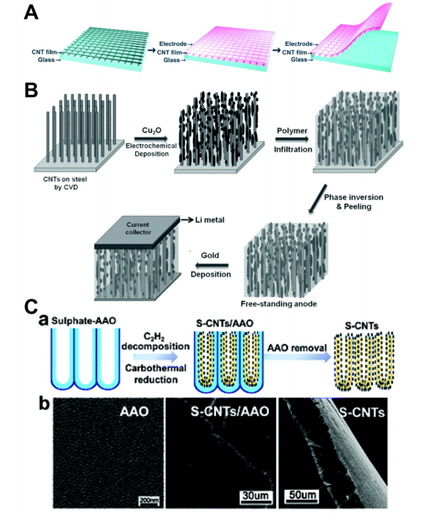
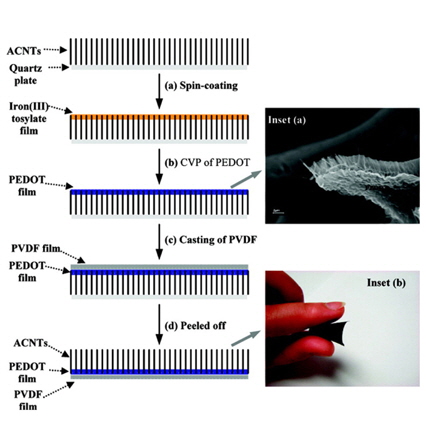
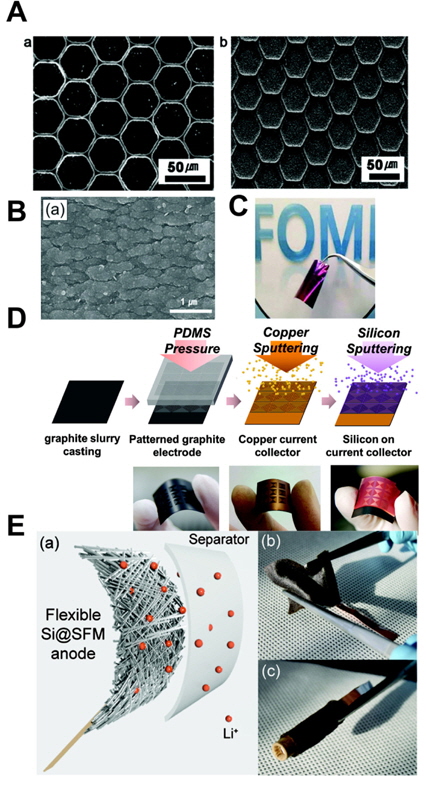
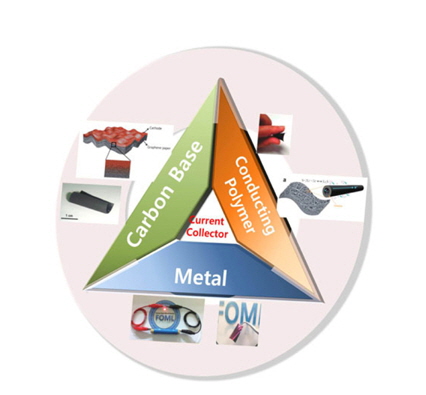
[1] S.-Y. Lee, K.-H. Choi, W.-S. Choi, Y. H. Kwon, H.-R. Jung, H.-C. Shin and J. Y. Kim,
Energy Environ. Sci.,
2013,
6, 2414.

[2] Y. Hu and X. Sun,
J. Mater. Chem. A,
2014,
2, 10712.

[3] K. Xie and B. Wei,
Adv. Mater.,
2014,
26, 3592.

[4] X. Wang, X. Lu, B. Liu, D. Chen, Y. Tong and G. Shen,
Adv. Mater.,
2014,
26, 4763.

[5] Q. Sa and Y. Wang,
J. Power Sources,
2012,
208, 46.

[6] Y.-L. Kim, Y.-K. Sun and S.-M. Lee,
Electrochim. Acta,
2008,
53, 4500.

[7] G.-W. Lee, J. H. Ryu and S. M. Oh,
J. Korean Electrochem. Soc.,
2010,
13, 157.

[8] J. Zhu, J. Feng and Z. Guo,
RSC Adv.,
2014,
4, 57671.

[9] C. Iwakura, Y. Fukumoto, H. Inoue, S. Ohashi, S. Kobayashi, H. Tada and M. Abe,
J. Power Sources,
1997,
68, 301.

[10] M. S. Yazici, D. Krassowski and J. Prakash,
J. Power Sources,
2005,
141, 171.

[11] K. Wang, S. Luo, Y. Wu, X. He, F. Zhao, J. Wang, K. Jiang and S. Fan,
Adv. Funct. Mater.,
2013,
23, 846.

[12] H. Lin, W. Weng, J. Ren, L. Qiu, Z. Zhang, P. Chen, X. Chen, J. Deng, Y. Wang and H. Peng,
Adv. Mater.,
2014,
26, 1217.

[13] L. Hu, J. W. Choi, Y. Yang, S. Jeong, F. La Mantia, L.-F. Cui and Y. Cui, In: Proc. Natl. Acad. Sci. U.S.A;
2009, 106, pp 21490.

[14] L. Hu, H. Wu, F. La Mantia, Y. Yang and Y. Cui,
ACS Nano,
2010,
4, 5843.

[15] S.-L. Chou, Y. Zhao, J.-Z. Wang, Z.-X. Chen, H.-K. Liu and S.-X. Dou,
J. Phys. Chem. C,
2010,
114, 15862.

[16] X. Li, J. Yang, Y. Hu, J. Wang, Y. Li, M. Cai, R. Li and X. Sun,
J. Mater. Chem.,
2012,
22, 18847.

[17] A. Goyal, A. L. M. Reddy and P. M. Ajayan,
Small,
2011,
7, 1709.

[18] X. Jia, C. Yan, Z. Chen, R. Wang, Q. Zhang, L. Guo, F. Wei and Y. Lu,
Chem. Commun.,
2011,
47, 9669.

[19] G. Zhou, D.-W. Wang, F. Li, P.-X. Hou, L. Yin, C. Liu, G. Q. Lu, I. R. Gentle and H.-M. Cheng,
Energy Environ. Sci.,
2012,
5, 8901.

[20] H. Gwon, H.-S. Kim, K. U. Lee, D.-H. Seo, Y. C. Park, Y.-S. Lee, B. T. Ahn and K. Kang,
Energy Environ. Sci.,
2011,
4, 1277.

[21] G. Ning, C. Xu, Y. Cao, X. Zhu, Z. Jiang, Z. Fan, W. Qian, F. Wei and J. Gao,
J. Mater. Chem. A,
2013,
1, 408.

[22] J.-Z. Wang, C. Zhong, S.-L. Chou and H.-K. Liu,
Electrochem. Commun.,
2010,
12, 1467.

[23] J. Liang, Y. Zhao, L. Guo and L. Li,
ACS Appl. Mater. Interfaces,
2012,
4, 5742.

[24] J. Jin, Z. Wen, G. Ma, Y. Lu, Y. Cui, M. Wu, X. Liang and X. Wu,
RSC Adv.,
2013,
3, 2558.

[25] X. Huang, B. Sun, K. Li, S. Chen and G. Wang,
J. Mater. Chem. A,
2013,
1, 13484.

[26] C. Uthaisar and V. Barone,
Nano Lett.,
2010,
10, 2838.

[27] X. Zhao, C. M. Hayner, M. C. Kung and H. H. Kung,
ACS Nano,
2011,
5, 8739.

[28] N. Li, Z. Chen, W. Ren, F. Li and H.-M. Cheng, In: Proc. Natl. Acad. Sci. U.S.A;
2012, 109, pp 17360.

[29] G. A. Snook, P. Kao and A. S. Best,
J. Power Sources,
2011,
196, 1.

[30] K. Naoi and M. Morita,
Electrochem. Soc. Interface,
2008,
17, 44.


[31] J.-Z. Wang, S.-L. Chou, J. Chen, S.-Y. Chew, G.-X. Wang, K. Konstantinov, J. Wu, S.-X. Dou and H. K. Liu,
Electrochem. Commun.,
2008,
10, 1781.

[32] L. Noerochim, J.-Z. Wang, D. Wexler, M. M. Rahman, J. Chen and H.-K. Liu,
J. Mater. C.,
2012,
22, 11159.

[33] J. Chen, Y. Liu, A. I. Minett, C. Lynam, J. Wang and G. G. Wallace,
Chem. Mater.,
2007,
19, 3595.

[34] L. Nyholm, G. Nyström, A. Mihranyan and M. Strømme,
Adv. Mater.,
2011,
23, 3751.

[35] M.-H. Park, M. Noh, S. Lee, M. Ko, S. Chae, S. Sim, S. Choi, H. Kim, H. Nam, S. Park and J. Cho,
Nano Lett.,
2014,
14, 4083.

[36] J.-Y. Choi, D. J. Lee, Y. M. Lee, Y.-G. Lee, K. M. Kim, J.-K. Park and K. Y. Cho,
Adv. Funct. Mater.,
2013,
23, 2108.

[37] S. W. Kim, J. H. Yun, B. Son, Y.-G. Lee, K. M. Kim, Y. M. Lee and K. Y. Cho,
Adv. Mater.,
2014,
26, 2977.

[38] S. Song, S. W. Kim, D. J. Lee, Y.-G. Lee, K. M. Kim, C.-H. Kim, J.-K. Park, Y. M. Lee and K. Y. Cho,
ACS Appl. Mater. Interfaces,
2014,
6, 11544.