1. Introduction
A rapid response to energy demands is the most attractive feature of electrochemical double-layer capacitors (EDLCs) [1,2]. Quaternary ammonium tetrafluoroborate (QA BF4) salts are often used in the nonaqueous electrolytes employed in EDLCs because QA BF4 salts are inexpensive and highly soluble in nonaqueous solvents, conferring a high conductivity on such solvents [3]. Of the various QA BF4 sal ts avai lable, tet raethylammonium tetrafluoroborate (TEA BF4) is the most widely used electrolyte in EDLCs. In addition, TEA BF4 shows reliable performance and good electrochemical durability in EDLCs. 5-Azonia-spiro[4.4]nonane tetrafluoroborate (C8 in Fig. 1) has a molecular weight similar to that of TEA BF4 but exhibits a spiro bicyclic structure. Further, these two structures have almost the same cation size. However, EDLCs based on C8 exhibit higher capacitances and better electrochemical stabilities than do those based on TEA BF4 [4-8]. This is because spiro salts are bicyclic structures, wherein the rotating, stretching, and bending modes of the alkyl branches are limited. In contrast, the alkyl chains of TEA BF4 can bend, rotate, and stretch freely.
In this work, two pairs of cyclic (or bicyclic) and acyclic ammonium tetrafluoroborate salts are prepared. The cations of each pair have nearly identical molecular weights. Therefore, the only difference in the cations in each pair is in terms of the structure, that is, whether it is cyclic or not. The effects of the cyclic cation structure on cell capacitance are discussed. The capacitance of an EDLC is dependent on the length of the alkyl chains in the electrolyte used [5,9,10]. Therefore, to minimize the difference in the ion sizes, an identical number of carbon atoms are used in the pairs of cyclic and acyclic cations. The structures of the cations employed in this study are shown in Fig. 1. As the ion size increases, so does the electrolyte viscosity. Hence, large cations are unsuitable for use in EDLCs. Therefore, in this study, the ions chosen were as small as possible.
2. Experimental Section
2.1. Chemicals
5-Azonia-spiro[4.4]nonane tetrafluoroborate (C8, a cyclic structure with eight carbons) was synthesized by the following procedure. First, pyrrolidine (Alfa Aesar, 98%), 1, 4-dibromobutane (Alfa Aesar, 98%), and anhydrous potassium carbonate (Daejung Chemicals & Metals Co., 99.5%) were mixed in a 1:1.2:1.2 molar ratio in isopropyl alcohol (Daejung Chemicals & Metals Co.) and stirred for 12 h at 80℃. The solid salts (KBr and the bicarbonate and carbonate salts) were filtered out, and the solvent and any unreacted components were removed under vacuum. A yellow liquid was obtained, from which an ammonium halide was precipitated using acetone (Daejung Chemicals & Metals Co.). The obtained intermediate and HBF4 (50% in H2O, Alfa Aesar) were reacted in methanol in a 1:1.1 mole ratio for 12 h. The final product was recrystallized from n-butanol before use. A few drops of a 0.1 M aqueous solution of AgNO3 (Daejung Chemicals & Metals Co.) were added to the product solution to observe whether precipitation with halide ions occurred; this would have indicated the presence of intermediate impurities in the final product. Nuclear magnetic resonance (NMR, Bruker Avance-250, 250 MHz) measurements were performed to identify the final products. The solvents for the NMR measurements were dimethyl sulfoxide-d6 and CDCl3 (Cambridge Isotope Laboratories, Inc., DMSO-d6).
The rest of the ammonium salts were prepared in the same manner from the following starting materials. The materials used for the synthesis of 5-azoniaspiro[4.5]decane tetrafluoroborate (C9, a cyclic structure with nine carbons) were pyrrolidine, 1,5-dibromopentane (Alfa Aesar, 98%), and anhydrous potassium carbonate. 6-Azonia-spiro[5.5]undecane tetrafluoroborate (C10, a cyclic structure with ten carbons) was prepared from piperidine (Alfa Aesar, 98%), 1,5-dibromopentane, and anhydrous potassium carbonate. For the recrystallization of C10, an additional extraction process, performed using a separatory funnel, was necessary. Dichloromethane (Daejung Chemicals & Metals Co.) and deionized water were used in a 10:0.5 volumetric ratio, and C10 was obtained from the dichloromethane layer. 1,1-Dimethylpyrrolidinium tetrafluoroborate (C6, a cyclic structure with six carbons) was prepared from 1-methyl pyrrolidine and iodomethane (Tokyo Chemical Industry Co. Ltd, 98.0%). Diethyldimethylammonium tetrafluoroborate (C6O, the structure is similar to that of C6 but open) was prepared from dimethylethylamine (Tokyo Chemical Industry Co. Ltd, 98.0%) and bromoethane (Tokyo Chemical Industry Co. Ltd, 98.0%). Tetraethylammonium tetrafluoroborate (C8O) was prepared from triethylammonium chloride and HBF4 (50% in H2O, Alfa Aesar). The structures of the prepared salts are shown in Fig. 1. All the products were dried under vacuum for 2 days in order to remove as much water as possible.
The results of the 1H-NMR (250 MHz) measurements are as follows: C8O (DMSO-d6) : δ 1.16 (3H, t), 3.18 (2H, q); C8 (DMSO-d6) : δ 2.06 (4H, m), 3.47 (4H, m); C9 (DMSO-d6) : δ 1.54 (2H, m), 1.77 (4H, m), 2.04 (4H, m), 3.31 (4H, m), 3.47 (4H, m); C10 (DMSO-d6) : δ 1.522 (2H, m), 1.731 (4H, m), 3.364 (4H, m); C6O (DMSO-d6) : δ 1.22 (6H, m), 2.94 (6H, s), 3.27 (4H, q); C6 (DMSO-d6) : δ 2.09(4H, q), 3.08(6H, s), 3.44(4H, m). The NMR spectra of all the synthesized salts are shown in Fig. S1, while the yields of the products after recrystallization are listed in Table S1.
2.2. Cell preparation
The active material used for the EDLC electrodes was activated carbon (AC, MSP 20, Kansai Coke). The electrodes were prepared from styrene-butadiene rubber (SBR, Zeon, BM-400B) and a carboxymethylcellulose (CMC, Sigma-Aldrich) emulsion binder containing Super-P black (MMM, Belgium) as the conducting agent. The active material/Super-P black/SBR/CMC ratio was 81:12.8:4.2:2. The thickness of the prepared electrodes was 200 μm. The electrodes were punched to produce discs with a diameter of 14 mm, and the discs were dried in a vacuum oven. The salts were dissolved in acetonitrile (AN, J. T. Baker; 99.8%) to obtain 1.0 M electrolyte solutions. Cell tests were performed using 2032-type coin cells (Hohsen Co.). Two carbon electrodes were used for the full-cell tests. A 40-μ-thick cellulose separator was placed between the two electrodes. The cells were assembled in a dry box.
2.3. Instruments
A cell cycler (PNE Solution Co.) was used for the galvanostatic cell tests. Cyclic voltammetry (CV) measurements were performed using the coin cells between 0 and 3.0 V. A Swagelok type cell was used to perform linear sweep voltammetry (LSV) measurements. A silver wire served as the reference electrode for these measurements. The performances of the cells were evaluated through galvanostatic charge/discharge measurements. Initially, the cells were charged to 3.0 V at a constant current. Then, a constant potential of 3.0 V was maintained until the current was 1/10 of the charge current. Discharging started immediately after the constant-potential charging mode. The discharge current was the same as the charge current. The cut-off potentials for the discharge and charge cycles were 0 and 3.0 V, respectively. The cell capacitance was determined using the following equation:
The cell capacitance (in farads) was calculated from the galvanostatic charge/discharge measurement data, that is, from the current, I (in amperes (A)), and the ΔV/Δt value, which was obtained from the slope of the voltage versus time curves. The slope from 0 to 3.0 V was used to calculate the discharge capacitances from the galvanostatic tests. The capacitance values were averaged over five measurements. The specific capacitance, Cam (in farads per gram of electrode; F g−1), values of the samples were determined using Eq. (2), where mam is the mass of AC in the electrode [11].
In a symmetrical cell, the mass of each electrode is almost the same. The value of mam was obtained from the average mass of AC in each electrode in the cell.
Conductivity measurements were performed using a conductivity meter (Mettler Toledo, Seven Go Pro SG7-FKS) at several temperatures. The four-electrode conductivity probe (Mettler Toledo, SevenGo Pro Inlab 738) used was calibrated with a standard solution (conductivity of 12.88 mS cm−1; Mettler Toledo) before the measurements. Further, after each use, the probe was cleaned with nitric acid, rinsed with deionized water, and dried. The viscosities of the electrolytes were measured using capillary viscometers (SI Analytics GmbH, 527 10 and ViscoClock). The average values of three measurements were taken. The conductivity and viscosity measurements were performed in a dry box. The N2 (99.999%) adsorptiondesorption isotherms of AC were obtained using a gas analyzer (MicrotracBEL Corp., BELSORP-max). Before analysis, the AC sample was outgassed at 300℃ for 4 h under vacuum. The surface area was determined by the Brunauer-Emmett-Teller method. The total pore volume and surface area were measured between relative pressures of 0 and 0.05. From the measurements, it was found that 90.2% of the pores were micropores with diameters of less than 2 nm, with the average surface area being 2135.1 m2 g−1.
3. Results and Discussion
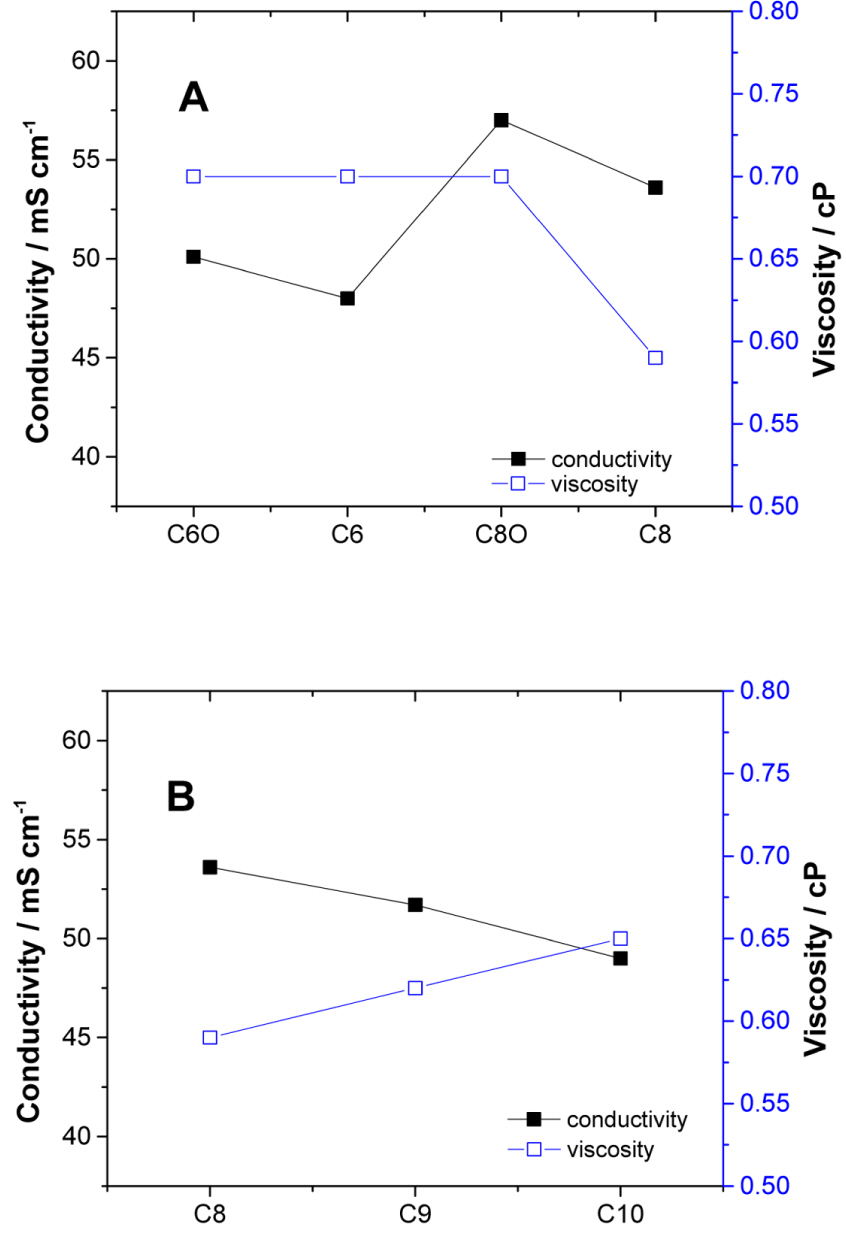
The C6 (C6 and C6O) and C8 (C8 and C8O) pairs were synthesized to compare the behaviors of cyclic and acyclic ammonium tetrafluoroborate salts during EDLC operation. The spiro-ammonium tetrafluoroborate salts C8, C9, and C10 are bicyclic structures, which exhibit limited vibrational and rotational modes. The cation size in this group of spiro-ammonium salts increases gradually as one moves from C8 to C10. The conductivities and viscosities for the C6 and C8 pairs are compared in Fig. 2A. The conductivities of the electrolytes based on the C6 pair are lower than those of the electrolytes based on the C8 pair. Larger ions have smaller solvation radii; this is a plausible reason for the conductivities of the C8 electrolytes being higher than those of the C6 electrolytes. Further, the viscosities of the C8 electrolytes are much lower than those of the other electrolytes. However, the spiro-ammonium ion (C8) moves more slowly in the bulk solution than does the corresponding acyclic ion (C8O). The spiro-ammonium salts C8 to C10 have significantly lower viscosities (see Fig. 2B) than those of the C6, C6O, and C8O salts. However, the low viscosities of the spiro-salt electrolytes do not result in their conductivities being higher than that of C8O. The viscosity (and conductivity) versus ion size relationship is a linear one for spiro salts. Further, for spiro salts, an increase in the cation size results in the viscosity being higher and the conductivity being lower than those in the case of smaller cations. As the ion size increases, ion movement through the solvent slows, and the viscosity of the solution begins to play a critical role in determining the conductivity.[12] The spiro salts shown in Fig. 2B are simple structures whose conductivity (or viscosity) can be predicted based on the cation size. In contrast, the size of the cation and knowing whether the structure is cyclic or acyclic does not allow one to predict the conductivity of the electrolytes (C6, C6O, C8, and C8O) shown in Fig. 2A. The linear alkyl groups in C6, C6O, and C8O have more conformational freedom (rotating, bending, and stretching), making it more difficult to predict their ion conductivities than is the case for spiro salt electrolytes.
Fig. 2.
Conductivities and viscosities of 1.0 M solutions in AN of (A) acyclic and cyclic salts and (B) spiro salts.
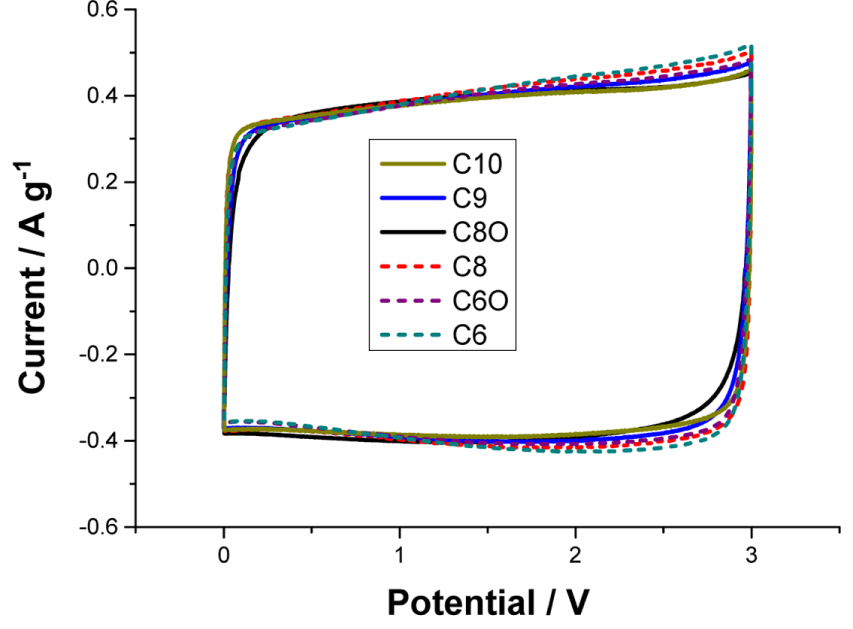
The CV curves for all the electrolytes were obtained, in order to observe whether any faradaic reactions occurred in the electrolytes (Fig. 3). The scans were performed between 0 and 3.0 V at a scan rate of 5 mV s−1. Within this range, only capacitive currents were observed in the CV curves. The differences in the conductivities and viscosities of the electrolytes were not large enough for them to affect the overall differences in the current values. The current values at each voltage were slightly different and depended on the movement of the cations during the charging/discharging states. In the case of electrolytes C6, C8, and C6O, the current values remained high during the later stages of the charge cycle. These electrolytes also responded rapidly during the discharge cycle, as evidenced by the high discharge currents in the early stages of the discharge cycle. This CV curve feature is characteristic of small and mobile cations [13]. The LSV curves obtained using the electrolytes (Fig. S2) indicate that the potential windows for the electrolytes are similar; the exception is C6O, for which the potential window is slightly narrower. The potential windows for all the electrolytes are wider than 3.0 V, which is the width of the cut-off potential for the charge process. The cycle life tests (additional stability test), whose results are shown in Fig. S3, indicated that the cells exhibited stable EDLC operation for up to 5000 cycles. This confirmed that the cells operated without undergoing severe electrolysis.
The capacitances of the cells based on the electrolytes were determined from the curves for the galvanostatic charge/discharge measurements (Fig. S4). The capacitance values were calculated from the slope between 0 and 3.0 V. As can be seen from Fig. 4, at low discharge currents, the capacitances are comparable. Further, at a current density of 0.1 A g−1, the discharge slopes for all the electrolytes are similar (Fig. S4A). As the current is increased, the ions that can move in and out of the pores readily are more effective in maintaining the capacitance than are the slow-moving ions. A current density of 5 A g−1 was sufficient to separate the ions in the order of their response rates in an electric field. In the case of the C6 and C8 pairs, the ions in C6 and C8 responded to the electric field more rapidly than did those in C6O and C8O, respectively. The cyclic ions (C6 and C8) required more time to discharge at 5 A g−1 (Fig. S4B) as compared to their acyclic counterparts (C6O and C8O); this was in keeping with the results shown in Fig. 4. Furthermore, we found that the conductivity and viscosity are not useful parameters for explaining why the capacitances of the cyclic salts are higher than those of the acyclic salts. Both C8 and C6 exhibit conductivities lower than those of their counterparts (C8O and C6O, respectively). The capacitances can be arranged as follows: C6 > C6O > C8 > C8O. Thus, the electrolytes containing small and cyclic ions have higher capacitances than those with large and acyclic ions. The acyclic C6O and C8O cations have two and four additional free linear alkyl groups, respectively, as compared to the C6 and C8 cations. These linear alkyl groups can bend and stretch freely, owning to which the corresponding ions have a larger effective radii than those of the C6 and C8 cations. The smaller effective radii of the cyclic ions is advantageous, allowing for faster ion transfer in the pores and resulting in a higher capacitance. Furthermore, for the spiro ammonium salts, the capacitance at 5 A g−1 is inversely proportional to the cation size. Consequently, it can be concluded that the capacitance is related not to the conductivity but to the ionic size.
Fig. 4.
Discharge capacitance vs. current density curves. The capacitance values are the averages of five measurements.
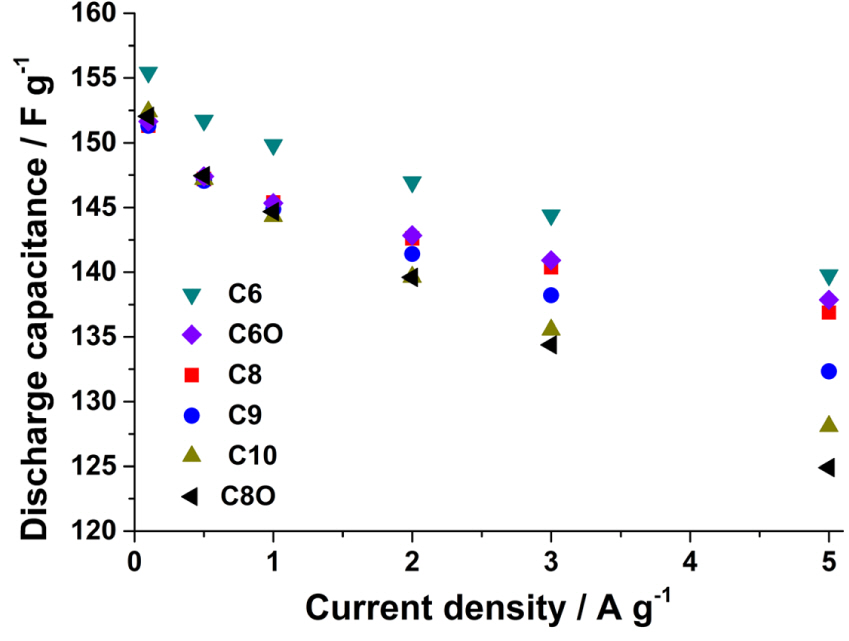
The conductivity is affected by the size of the ion, the structure of the alkyl chain, and the viscosity of the electrolyte solution. We did not observe a relationship between the conductivity and capacitance [13,14] For example, the conductivity of C6 is 10% lower than that of C8, as shown in Fig. 2. In addition, the conductivity is a measure of the movement of ions in the bulk solution, in which the ions are solvated. However, the capacitance is governed by the movement of the ions in the pores in AC. The ions become desolvated in the pores. Hence, the values obtained in the case of the bulk solution are not directly related to the ionic behavior in the very narrow pores.
The surface area of the AC sample used in this study was very large, and the sample primarily contained micropores. If an AC sample with fewer micropores than the one used in this work were to be used, the capacitance would probably not be directly dependent on the ion size. If meso- and macropores are present in the AC sample used, the solvated ions can enter these wide pores. As a greater number of solvation shells and solvent molecules enter the pores, they bring the properties of the bulk solution (conductivity and viscosity of the electrolytes) into the pores to some extent. In such cases, the capacitance will not be dependent on the ion size because the properties of the solvated ions and the solution will have a greater effect on the capacitance.
In the case of high-discharge-power applications, an AC sample with a greater number of mesopores may exhibit results that deviate from those presented here. In addition, salts that exhibit poor solubility or poor ionization behave differently from QA BF4 salts; QA BF4 salts are highly soluble and ionizable in typical EDLC solvents, whereas salts that are not readily ionized but form ion pairs in solution respond slowly to the electric field.
4. Conclusions
The capacitance of an EDLC is not dependent on the conductivity or viscosity of the electrolyte used but on the cation size. Although both the conductivity and the viscosity are important parameters affecting cell operation, small differences in these parameters do not mean that there will be a difference in the movement of the respective ions in the micropores. The electrolytes reported here exhibited a difference of only 20% in their conductivities; a difference this small in the conductivities of the bulk solutions has not effect on ion movement in the pores. In addition, conductivity is a bulk property and is related to the movement of the ions from the bulk solution to the surface of the electrode. The differences in electrolyte conductivity are not large enough to result in differences in the capacitance. In addition, the ions attracted to the electrode surface undergo desolvation as the charge potential increases. The desolvated ions exhibit properties that are different from those of the ions in the bulk solution. In the pores, the size of the desolvated ions becomes important, affecting the movement of the ions in the electric field. Because the linear alkyl groups of acyclic ammonium ions can bend and stretch freely, the effective cation radius of acyclic ammonium ions is larger than that of the corresponding cyclic ions. Therefore, the electrolytes based on C6 and C8 (cyclic and bicyclic structures) have higher capacitances than those based on C6O and C8O (the corresponding acyclic structures), respectively.