1. Introduction
Microbiologically Influenced Corrosion (MIC) or Bio-corrosion is the type of corrosion in which micro-organisms control the dissolution rate of materials [1]. Microorganisms could enhance metals dissolution either by utilizing or producing by-products i.e. sulphides, acids and by generating oxidizing species. These secretions or by product reduce the resistance of protective oxide layer of the metal/alloy. Microorganisms attach to the metal surface due to extra polymeric secretions and form a complex biofilm. This biofilm may promote or decrease the corrosion rate [2]. It has been proved in the previously published work that biofilms formed by the microorganisms may allow the ingress of chloride ions that may accumulate and result in the localized dissolution of metal at the biofilm/metal interface [3ŌĆō5]. In closed systems, i.e. water lines, chillers and heat exchangers etc., the microorganism strains can also reduce the aromatic and aliphatic hydrocarbons of the corrosion inhibitor molecules and could deteriorate their inhibitive performance [6]. Until the present day, MIC is a concerning for many industries i.e. the aviation fuel industry, nuclear power plants, water treatment plants, oil and gas pipeline networks etc. [7].
Pseudomonas aeruginosa is an aerobic and gram-negative bacterium with rod shape morphology. It is mostly found in drinking water, gas and oil reserves. It uses medium and long-chain alkanes present in organic fuels for their growth and metabolism [8]. As Pseudomonas aeruginosa is an aerobic bacterium, so their common mechanism in MIC is formation of differential oxygen cells on the metal surface. The depletion of oxygen concentration at the biofilm/ metal interface accelerates the dissolution of metal due to the formation of differential aeration cells across the biofilm. The biofilm conducts the diffusion of aggressive anions such as chlorides on the anodic sites and may initiate pitting under the biofilm [9,10].
316L stainless steel shows good corrosion resistance properties due to the formation of native oxide layer of chromium oxide (Cr2O3) on its surface. The break-down of this oxide passive film by the microorganisms or chloride ions could results in the localized dissolution of the substrate [11]. It has been reported that the progress of bacterial growth on the passive film could result in the formation of biofilm which may initiate localized pitting corrosion [12,2]. Morales et al. [2] studied the effect of Pseudomonas aeruginosa in 0.5 M NaCl neutral buffered media on 304 SS for 7 days of exposure and due to the formation of acidic metabolites.
Brass is a copper-zinc alloy and is mostly used in automotive industries, heat exchangers and water-cooling systems [13]. Brass, when exposed to the aqueous medium develops a compact oxide layer that protects its surface from general corrosion. However, in the presence of chloride ions in water increase, the risk of localized corrosion reactions and accelerated pitting or preferential leaching of the Zn rich phase is observed. In highly alkaline conditions, brass represents good pitting resistance due to the formation of zinc oxide ZnO in the native oxide layer [14ŌĆō18]. Previous studies suggested that in the presence of microorganisms different corrosion mechanism occur on the copper and copper alloy surfaces due to the formation of differential aeration cells, selective leaching, under deposit corrosion and cathodic depolarization etc. [14,19]. Mostly, the active-passive alloys i.e. stainless steels and copper base alloys under neutral or near-neutral conditions are degraded due to the occurrence of localized MIC [20]. Xiaodong et al. [21] studied the effect of Pseudomonas aeruginosa, sulfate reducing bacteria and their mixture on the corrosion properties of brass during 12 days of exposure and conclude that sulfate reducing bacteria could induce severe corrosion compared to Pseudomonas aeruginosa. The later bacterial strain could produce an extracellular polymeric substance (EPS) layer on the surface of brass that could inhibit the corrosion process.
In this work, the effect of Pseudomonas aeruginosa strain ZK on the corrosion and mechanical properties of the brass and 316L stainless steel was investigated. The main objective of this study is to explore the active/passive behavior of these alloys in the presence of bacteria. Under anaerobic conditions, in the presence of Pseudomonas aeruginosa, the poor stability of the passive film could enhance the overall dissolution and could deteriorate the mechanical properties of the SS316L and ╬▒-brass keeping in mind their extensive used in the chemical process industry equipment i.e. radiators and heat exchangers.
2. Experimental
2.1 Bacterial Strain and Minimal salt medium Composition
The bacterial strain namely Pseudomonas aeruginosa strain ZK (Genbank accession number KC243318) was used in this research work. The strain was isolated from the mid-country industrial zone in Punjab, Pakistan as previously reported elsewhere [12]. The sterilize minimal salt medium was prepared by dissolving 1 g (NH4)2SO4, 0.2 g MgSO4┬Ę7H2O, 0.5 g K2HPO4, 0.01 g FeSO4┬Ę7H2O and 10 g of glucose in 1 litre of de-ionized water [12].
2.2 Preparation of Coupons
316L stainless steel and ╬▒-brass sheets having a thickness of 6 mm were purchase and used without any further thermal treatment. The chemical composition of these alloy is given in Table 1.
For microstructural analysis, hardness and electrochemical testing, the square shape test coupons of 1 cm2 were cut from the sheets. For tensile testing, the specimens were prepared according to ASTM standard E8 [22]. All test coupons were sequentially ground by using silicon carbide papers of different grit size (P60, P80, P120, P180, P220, P320, P400, P600, P800, P1000, P1200 and P1500) followed by successive polishing with diamond pastes of 9 ╬╝m, 4 ╬╝m, 2 ╬╝m and 0.5 ╬╝m size and to get a smooth mirror-like surface finish. These polished samples were then washed with 70% ethanol solution and rinsed in de-ionized water to avoid surface contamination.
2.3 Biocorrosion Study
To investigate the MIC, the polished samples of both alloys were exposed to Pseudomonas aeruginosa bacterial strains at 37┬░C for 7 days. The samples were exposed to the controlled medium (1200 mL of Minimal salt solution) and inoculated medium (Minimal salt solution containing Pseudomonas aeruginosa strain ZK). Polished coupons of 316L SS and ╬▒-brass were suspended in both the experimental systems separately with the help of nylon wire. Then these systems were placed in an incubator at 37┬░C for 7 days. After 7 days, the coupons were removed from each medium and processed for surface analysis, mechanical testing and electrochemical evaluation.
2.4 Surface Analysis
After 7 days of exposure to the controlled and inoculated media, the coupons were examined to observe the surface morphology and the density of the biofilm. To fix the biofilm on the surface the coupons were dipped in a 3% glutaraldehyde solution followed by consecutive dipping in ethanol solutions of variable concentrations (25%, 50%, 70%, 90% and 100%) as a per standard protocol [23]. To remove the biofilm from the 316L SS coupons, these were sonicated in 1 M nitric acid solution for 15 minutes at 60┬░C. Similarly, the ╬▒-brass coupons were sonicated in 2 wt.% NaCl solution for 10 minutes. The surface morphology of the test coupons before and after removal of biofilm were examined by using a scanning electron microscope (FE-SEM) (Vega Tescan Inc., USA) with 20 kV beam voltage.
The microstructural details of the coupons after 7 days of exposure to the controlled and inoculate media were also investigated. The 316L SS coupons were electrolytically etched in 10% oxalic acid solution with 5 V for 5 minutes, while ╬▒-brass was electrolytically etched in 40% H3PO4 solution by applying 2 V for 15 minutes [24]. The microstructure of the etched coupons was observed under metallurgical microscope (Leica Model DM 15000M, Germany).
2.5 Mechanical Testing
The tensile test specimens of both 316L SS and ╬▒-brass were separately exposed to the controlled and inoculated media for 7 days and tested by using universal testing machine (Instron, Norwood, MA, USA). The hardness of the coupons was measured after removal from the controlled and inoculated medium by Vicker Hardness tester (Qualitest, USA) under an applied load of 2.94 N and 15 s dwell time. To get reproducible and more reliable results, multiple indents were made at different regions of the test coupons and the average of the hardness values is reported.
2.6 Electrochemical Measurements
To prepare the working electrode of 316L SS and ╬▒-brass for electrochemical testing, the test samples of 1 cm2 were mounted on the copper plate (6 ├Ś 3 inches) to make electrical contact and the surface of the copper plate was masked by coating cold-curing epoxy resin to avoid electrolyte contact. Only 316L and ╬▒-brass samples were exposed to the controlled and inoculated media. Open circuit potential (Eoc) and potentiodynamic polarization scans were performed by connecting the electrochemical cell with a potentiostat (Gamry,1000E, USA). Three electrodes electrochemical paint cell was used for electrochemical testing. Graphite rod and silver-silver chloride (Ag/AgCl) (saturated KCl) electrode were used as counter and reference electrodes, respectively. The metal coupons were exposed to the controlled and inoculated media for two hours before measuring the Eoc for 500 seconds. The potentiodynamic polarization scans were obtained within ŌłÆ 0.5 V to + 1.5 V (vs Eoc) by applying a sweep rate of 2.5 mV.sŌłÆ1.
3. Results and Discussion
3.1 Surface Analysis
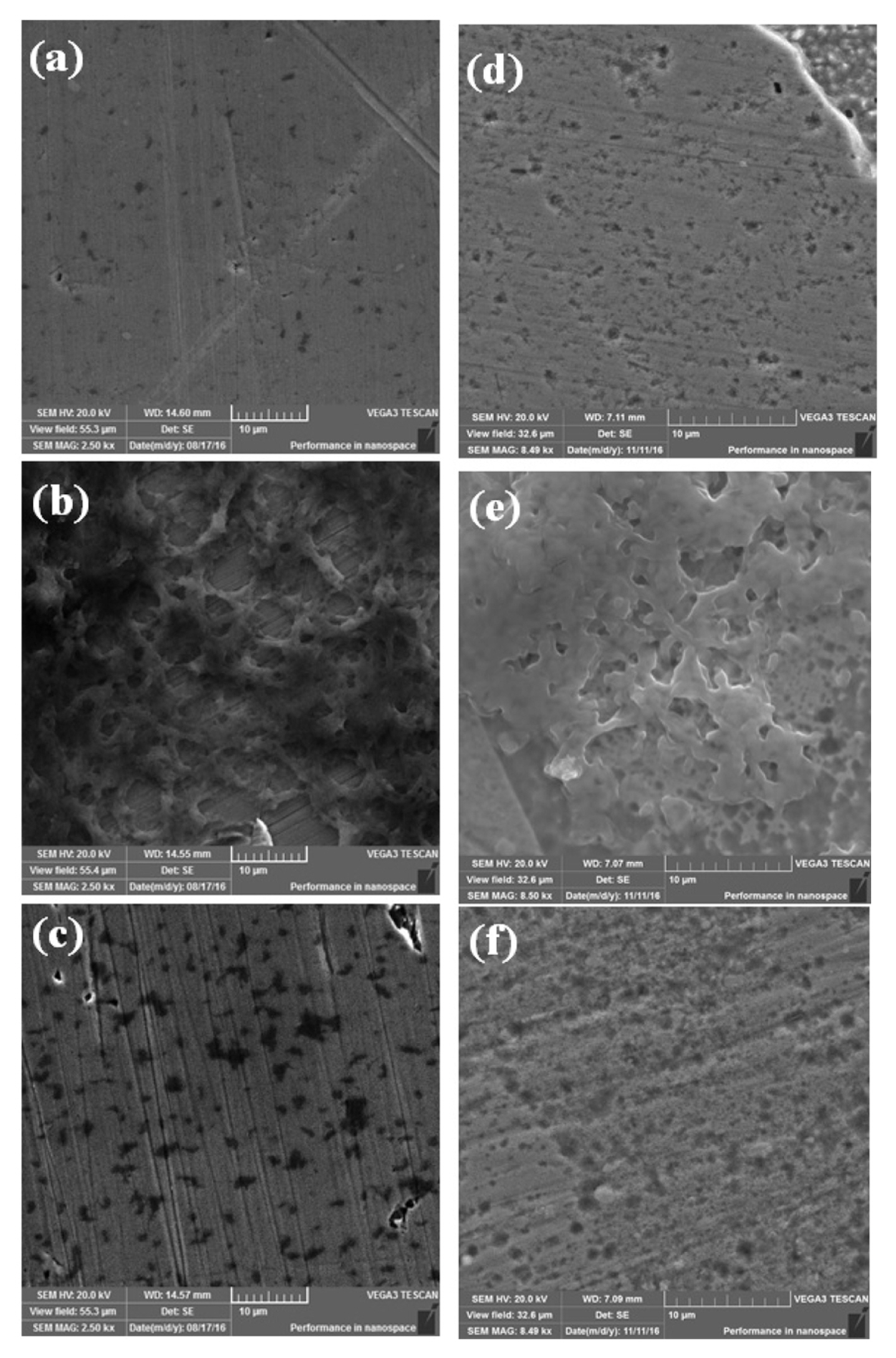
The surface morphology of the 316L SS and ╬▒-brass coupons were examined by SEM after 7 days of exposure to controlled and inoculated media as shown in Fig. 1. The pitting of the SS 316L was observed as shown in Fig. 1(a). The severe pitting of the SS 316L is expected due to the presence of chloride ions in the controlled medium [25,26]. However, in the presence of Pseudomonas aeruginosa strain ZK a biofilm was formed on the surface of 316L SS (Fig. 1 (b)), which could have restricted the ingress of ClŌłÆ towards the surface. As indicated, the bacterial colonies were attached to the surface and upon removal of the biofilm, the corrosion pits of variable size and depth were present on the surface as shown in Fig. 1 (c). The microstructure of ╬▒-brass experienced relatively large localized dissolution compared to 316L SS when exposed to the controlled medium for 7 days (Fig. 1 (d)). Upon exposure to the inoculated medium, the Pseudomonas aeruginosa strain ZK formed a relatively dense biofilm (Fig. 1 (e)) compared to the biofilm formed on the surface of 316L SS. After removing the biofilm, the presence of a large number of pits on the surface of ╬▒-brass indicated the aggressive attack of Pseudomonas aeruginosa as shown in Fig. 1 (f). The serve pitting of the ╬▒-brass in the presence of bacteria indicated its poor resistance to corrosion and the surface pits could deteriorate its mechanical properties as discussed below.
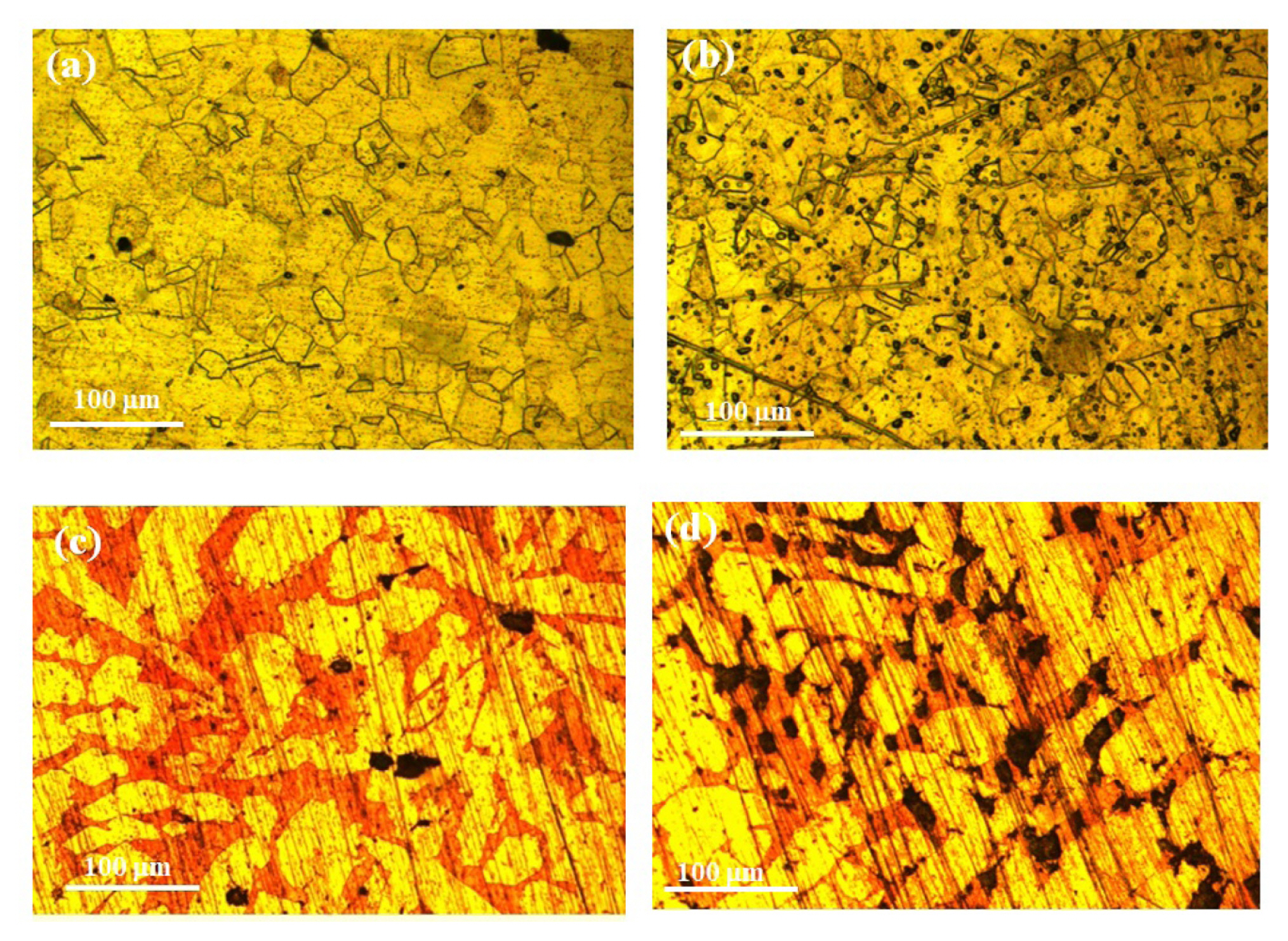
After exposure to the bacterial strains, the microstructural features of the 316L SS and ╬▒-brass was examined to estimate their dissolution tendency. Fig. 2 shows the microstructure of 316L and ╬▒-brass after the removal from the controlled and inoculated media. Fig. 2 (a) clearly shows that in the controlled medium, the pitting tendency of 316L SS was lower compared to the inoculated (Fig. 2 (b)). A large number of pits were formed within the austenite grains largely at the vicinity of grain boundaries and twin bands. The typical as-cast microstructure of ╬▒-brass consists of copper-rich dendrites (╬▒-phase) and a solution matrix composed of Zn enriched phase (relatively dark) as indicated in Fig. 2 (c). After exposure to the controlled and inoculated media, the presential dissolution of the Zn-rich phase was evident. The large-size irregular shape pits were formed in the Zn-rich phase and the pit density was found to be appreciably high after exposure to the inoculated medium as shown in Fig. 2 (d).
3.2 Post-exposure mechanical characterization
Fig. 3 shows the stress-strain curves of 316L SS and ╬▒-brass samples after exposure to both controlled and inoculated media. The mechanical properties i.e. yield strength, tensile strength and percentage elongation were determined as given in Table 2. The stress-strain curves of both samples presented minor influence on the mechanical properties of the 316L SS and ╬▒-brass after 7 days of exposure to the inoculated medium. The presence of Pseudomonas aeruginosa strain ZK adversely affected the surface morphology of both samples as discussed in the preceding section. The yield strength of 316 L SS is reported to be Ōēł 400 MPa [27] and after exposure to the controlled medium, this decreased slightly (396.3 ┬▒ 5 MPa). However, an appreciable decrease in the yield strength (331 ┬▒ 6 MPa) observed after exposure to the inoculated medium. The decrease in the yield strength was attributed to the enhanced localized surface dissolution due to bacterial attack. The formation of pits on the surface could act as stress concentration sites and the sample may deform at a lower stress level [28]. On the other hand, tensile strength (595.1 ┬▒ 4 MPa) and % elongation (16.9 ┬▒ 1%) of the 316L SS was negligibly affected after exposure to controlled and inoculated media.
Similar trends in the yield strength, tensile strength and % elongation values were observed in the case of brass samples. An appreciable decrease in the yield strength from 161.7 ┬▒ 2.0 MPa to 60.3 ┬▒ 4 MPa after exposure to controlled and inoculated media, respectively. In support of the microstructural features, the significant decrease in the yield strength was associated with the larger selective dissolution of the Znrich phase by the severe attack of Pseudomonas aeruginosa.
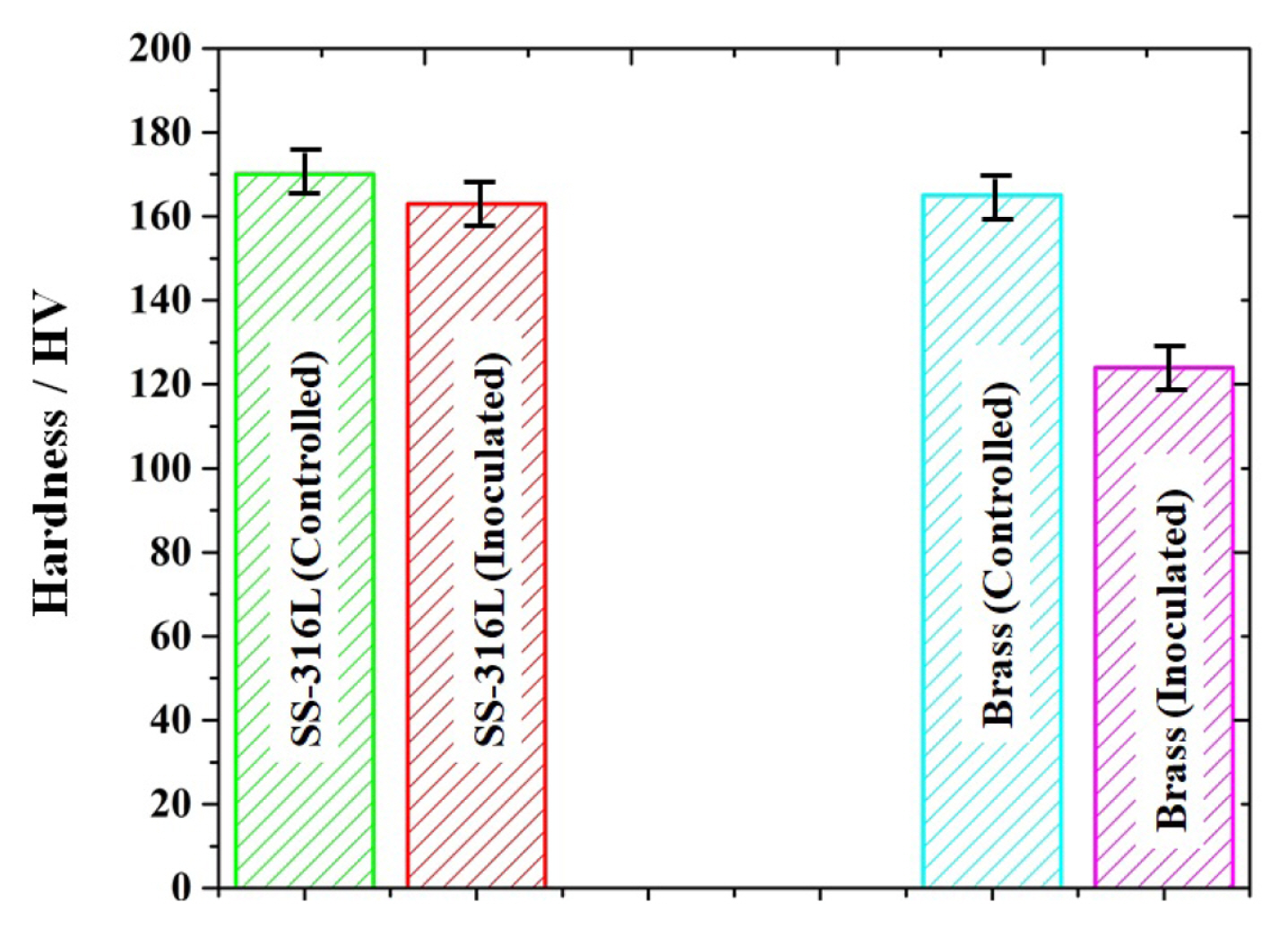
The hardness of both the samples was also measured after 7 days of exposure in controlled and inoculated media as depicted in Fig. 4. The presence of a large amount of salt concentration in the minimal solution (controlled medium) and the formation of biofilms in the inoculated medium severely deteriorated the surface finish. The hardness was measured on five different spots of each sample. The 316L SS sample exposed to a controlled medium presented a hardness of 170 ┬▒ 5 HV, which decreased 163 ┬▒ 3 HV in the presence of Pseudomonas aeruginosa. The ╬▒-brass samples showed a considerable decrease (from 165┬▒5 HV to 124┬▒5 HV) in hardness after 7 days of exposure to the bacterial strain.
3.3 Electrochemical Testing
The electrochemical behavior of 316L SS and ╬▒-brass open circuit polarization (OCP) and potentiodynamic polarization scans (PPS) in the inoculated medium. The OCP and PPS of both samples were obtained after exposure to the inoculated media for 7 days. Both 316L SS and ╬▒-brass presented negative OCP indicating their tendency to dissolve actively as validated from the immersion test results discussed above. For instance, the OCP of 316L SS was measured to be ŌłÆ230 mV vs. Ag/AgCl in the controlled medium and shifted to more negative (ŌłÆ420 mV vs. Ag/AgCl) in the inoculated medium, highlighting the adverse reaction of Pseudomonas aeruginosa on its surface. Similarly, the decrease in OCP of ╬▒-brass from ŌłÆ77 mV vs. Ag/AgCl (in controlled medium) to ŌłÆ188 mV vs. Ag/AgCl (in inoculated medium) corresponded to the possibility of active dissolution of Znrich phase in ╬▒-brass in the presence of bacterial strain as evaluated from the immersion tests (Fig. 2d). Fig. 5 shows the PPS curves of the 316L SS and ╬▒-brass after exposure to the inoculated medium for 7 days. Corrosion potential (Ecorr) and corrosion current density (icorr) were measured by extrapolating the PPS curves in the Tafel region. The relatively slow dissolution of the 316L SS can be validated from the lower icorr of the (0.0461 ╬╝A.cmŌłÆ2) compared to the ╬▒-brass (3.39 ╬╝A.cmŌłÆ2) as given in Table 3. The dissolution rate of these samples was also calculated from the icorr values and by using equation 1 [29].
Where;
The corrosion rates of the 316L SS and ╬▒-brass were calculated to be 5.14 ├Ś 10ŌłÆ4 mm/year and 7.15 ├Ś 10ŌłÆ2 mm/year respectively. The formation of passive film at potential > ŌłÆ0.2 V vs. Ag/AgCl on the surface of 316L SS was evident from the anodic polarization trend obtained in the inoculated medium. The 316L SS presented relatively negative Ecorr (ŌłÆ474 mV. Ag/AgCl) compared to compared to ╬▒-brass (ŌłÆ198 mV. Ag/ AgCl). 316L SS presented a low but almost constant passive current density (ipass) ~0.44 ╬╝A┬ĘcmŌłÆ2 suggesting the formation of a uniform passive film on its surface even in the presence of Pseudomonas aeruginosa strain ZK. However, in the case of ╬▒-brass, beyond 0.01 V vs. Ag/AgCl, the anodic current varied from 8.1 to 27.7 ╬╝A┬ĘcmŌłÆ2, which indicated its incapability to form the passive film in the inoculated medium. These results also confirmed the vigorous dissolution of ╬▒-brass in the presence of Pseudomonas aeruginosa compared to 316L SS.
As shown in Fig. 5, in the case of 316L SS, the accelerated increase in current density at potential > 0.74 V vs. Ag/AgCl represented the breakdown of the passive film further confirming the dissolution of the passive film by the bacterial strain. In other words, the passive film breakdown potential (Eb) of 316 L SS was increased to be ~747 mV vs. Ag/AgCl above which the rapid increase in current in current is associated with its aggressive dissolution. On the other hand, the ╬▒-brass presented Eb of ~1.14 mV vs. Ag/AgCl, which is possibly associated with the O2 evolution reaction (produced from H2O oxidation) in addition to its dissolution.
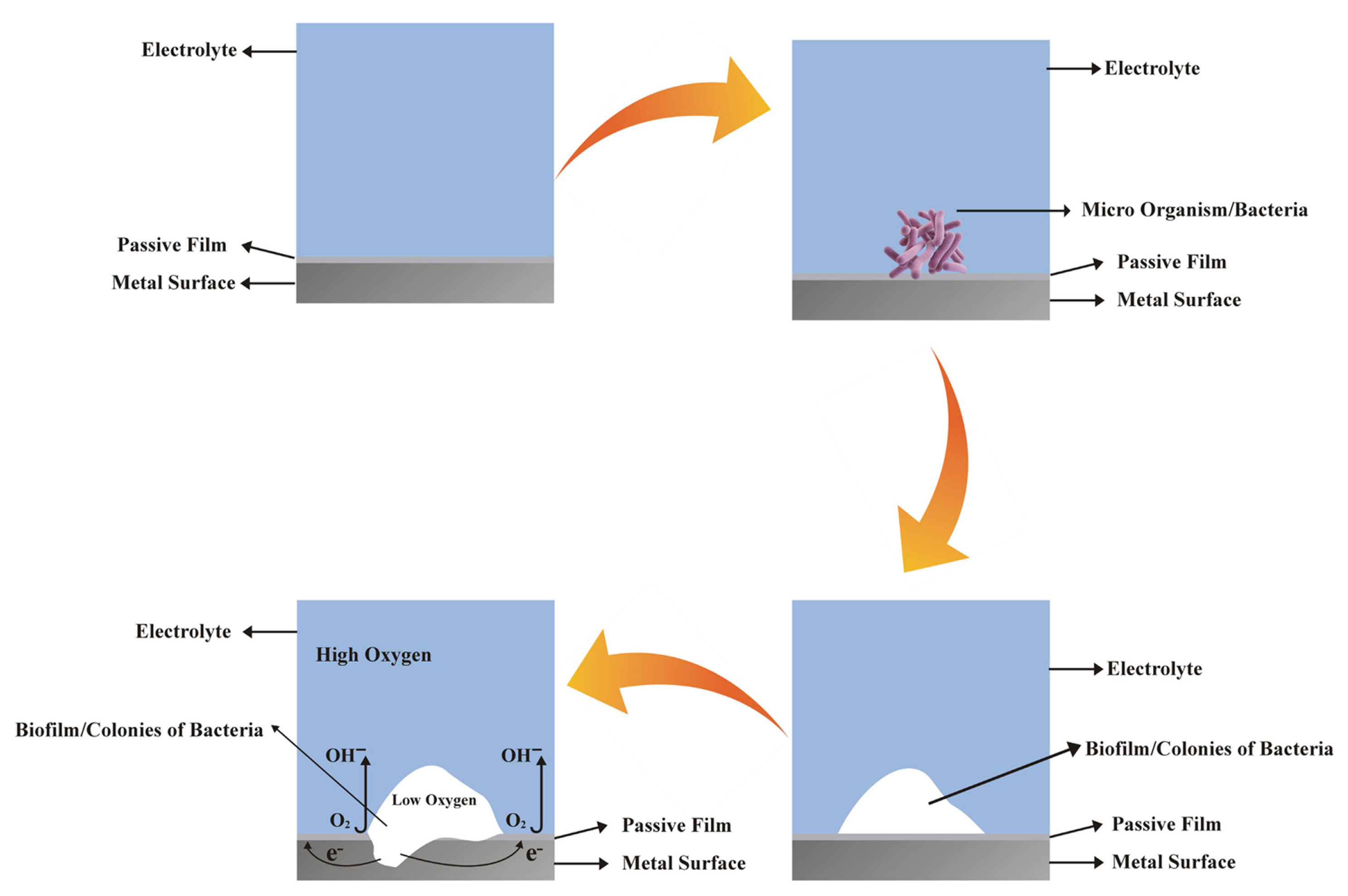
The corrosion mechanism of both the 316L SS and ╬▒-brass is also explained with the help of schematic diagram as shown in Fig. 6. The self-protective passive film is formed on the surface of 316L SS and ╬▒-brass when immersed in the inoculated medium. It is expected that when the bacteria first attach to the surface of metallic materials and tends to proliferate depending on the surface characteristics i.e. surface roughness, chemical composition and nature of the passive film [30ŌĆō33]. Due to the proliferation capability of the Pseudomonas aeruginosa strain ZK under aerobic conditions, the bacterium could grow on the surface of the alloys by forming the biofilm. The biofilm developed on the surface oxide film that may be dissociated locally by the oxygen-consuming bacteria. Due to the formation of biofilms, the dissolution may proceed due to the depletion of oxygen at the biofilm/substrate interface, which is essential for the stability and growth of the passive film. In other words, due to the depletion of oxygen under the biofilm, the anodic dissolution of the substrate would take place at the active site due to the passive film breakdown leading to pitting.
4. Conclusions
In the presence of Pseudomonas aeruginosa strain ZK, compared to 316L SS, a relatively dense biofilm was formed on the surface of ╬▒-brass leading to its serve preferential dissolution (pitting) during 7 days of exposure. A large density of irregular shape pits specially in the Zn rich phase was evident in ╬▒-brass compared to 316L SS., which presented pitting within the austenite grains but at the vicinity of grain boundaries and twin bands. An appreciable decrease in the yield strength of 316L SS (from 396.3 ┬▒ 5 MPa to 331┬▒ 6 MPa) and ╬▒-brass (from 161.7 ┬▒ 2.0 MPa to 60.3 ┬▒ 4 MPa) was observed after exposure to the inoculated medium, which is associated with the localized dissolution of these alloys due to bacterial attack. After 7 days of exposure to Pseudomonas aeruginosa strain ZK, relatively slow dissolution of the 316L SS compared to the ╬▒-brass was evaluated from the electrochemical tests. For instance, the corrosion rates of 316L SS and ╬▒-brass were 5.14 ├Ś 10ŌłÆ4 mm/year and 7.15 ├Ś 10ŌłÆ2 mm/year respectively. Also, in the case of 316L, the passive film stability in the presence of bacterial strain was evaluated from the low ipass (~0.44 ╬╝A┬ĘcmŌłÆ2) within 0.2 <E<0.74 V vs. Ag/AgCl potential range. However, the continuous increase in the anodic current (from 8.1 to 27.7 ╬╝A┬ĘcmŌłÆ2) at potential > 0.01 V vs. Ag/AgCl confirmed the poor corrosion resistance of ╬▒-brass.